

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v22.1.MS230102
Year: 2023, Volume: 22, Issue: 1, Pages: 27-36
Original Article
Kazi Asraf Ali ✉ 1 , Riya Das 2 , Sabyasachi Choudhuri 1
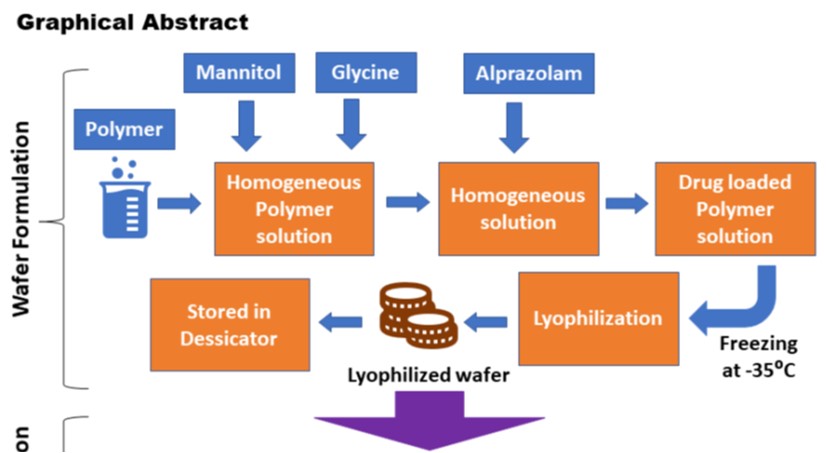
Our primary goal of this work was to create and test a mucoadhesive lyophilized rapid dissolving sublingual wafer of Alprazolam using a natural mucoadhesive agent extracted from black gram (Vigna mungo L.) seeds. We examined the pH, swelling volume, moisture absorption capability, mucoadhesive strength, and viscosity of the natural mucoadhesive agent. We compared it with synthetic mucoadhesive agents such as Hydroxypropyl cellulose (HPC) and Carbopol 934 (CP 934). The prepared wafers of both categories were characterized and compared for mechanical and texture properties, wetting time, disintegration time, Scanning Electron Microscopy (SEM), in vitro drug release, and ex vivo permeation study. We found that the pH of V. mungo mucilage (VMM) was 6.95±0.75, which lies between the normal sublingual mucosal range (pH 6-7), suggesting non-irritability to the mucosa. Attenuated total reflectance-Fourier-transform infrared (ATR-FTIR) peak showed no significant interaction between Alprazolam and mucoadhesive materials. The micrographs of SEM predicted good porosity of the wafer which leads to rapid wetting, disintegration, and dissolution. It is inferred from the study that the fast-dissolving wafer prepared from the VMM gave a better result than the HPC wafer in respect of various parameters. Hence, this study discovered an alternative method to deliver Alprazolam.
Keywords: Lyophilization, Permeability, Solid dosage form(s), Mucoadhesive, Texture
© 2023 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.