

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v22.1.MS230206
Year: 2023, Volume: 22, Issue: 1, Pages: 37-41
Original Article
Nasir Ibrahim ✉ 1 , Musa Aminu 2 , Abdullahi Musa Ismail 1 , Yusuf Amina Jega 1 , Awwalu Salisu 2
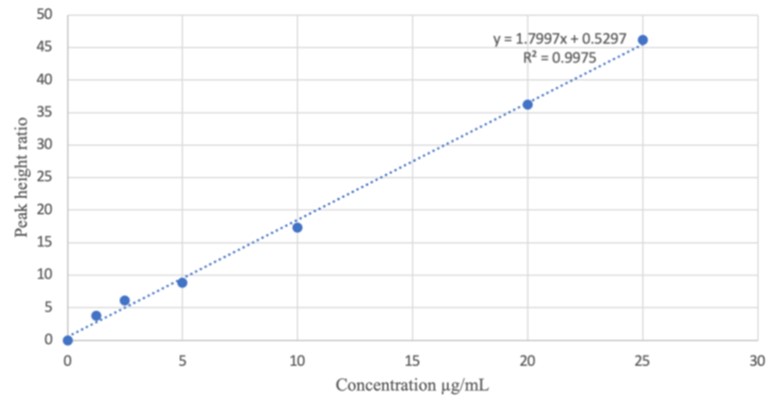
Several extraction steps involved while analyzing metformin in plasma necessitates the need to develop a simple less tedious RP-HPLC method for metformin analysis in human saliva.Blank saliva (2 mL) was spiked with 2 mL solution (12.5 µg mL-1) of metformin and 1 mL solution (0.5 µg mL-1) of caffeine as internal standard (IS). The mixture was vortex mixed and centrifuged at 3000 rpm for 10 minutes. A portion (0.5 mL) of the resultant solution was injected into the HPLC machine (Agilent 1260 infinity). The optimized conditions included a mobile phase of methanol:water (80:20 v/v) containing 0.1 % orthophosphoric acid, isocratic elusion mode, an injection volume of 10 µL, flow rate of 1 mLmin-1, at 35°C and detection wavelength of 232nm. Calibration curve(1.25to25.0 µg mL-1) was prepared by plotting the peak height ratios of metformin Vs IS against their corresponding concentrations. The method was validated according to ICH guidelines. Metformin and caffeine eluted at 1.6 and 2.6 minutes respectively. The method was precise (<1 % RSD), accurate (% Er of 1.00 and % recovery of 99.98 %) with linear calibration curve (r= 0.9987). The developed method can be used for determination of metformin in human saliva.
Keywords: Metformin, Saliva, Isocratic elution, RP-HPLC
© 2023 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.