

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 3, Pages: 178-188
Original Article
Shailesh B Patil1, Jitendra D More2,∗
1Department of Pharmaceutical Chemistry, DCS’s ARA College of Pharmacy, Nagaon, Dhule, University of KBC-NMU, Jalgaon, Maharashtra, India
2Department of Pharmaceutical Chemistry, Prof. Ravindra Nikam College of Pharmacy, Gondur, Dhule, University of KBC-NMU, Jalgaon, Maharashtra, India
*Corresponding author email: [email protected]
The evaluation of Naproxen Sodium Fast Dissolving Tablets emphasizes the critical role of careful formulation design and thorough testing in pharmaceutical development. Among the formulations, F7 demonstrated outstanding properties, including excellent flow characteristics and optimal tablet hardness. Stability testing over a four-week period showed consistent performance, though minor variations in tablet attributes were observed. Continuous monitoring is advised to ensure sustained efficacy. The in-vitro release profile revealed rapid disintegration and dissolution, crucial for prompt drug release. These results highlight the necessity of stringent quality control measures and ongoing optimization to enhance patient outcomes and ensure the reliability of Naproxen Sodium Fast Dissolving Tablets. In a parallel evaluation, the stability and performance of Naproxen Sodium Fast Dissolving Tablets were assessed under various environmental conditions, showing that temperature and humidity significantly impacted Naproxen Sodium concentration and dissolution rates. Formulation F7 emerged as the most promising, offering superior flow properties, compressibility, and dosage consistency. In-vitro tests confirmed rapid disintegration and dissolution, aligning with Pharmacopoeial standards. Four-week stability studies indicated consistent tablet characteristics, with only slight changes in hardness, friability, and drug content. Continued monitoring is recommended to ensure long-term stability and product quality. These findings underscore the importance of rigorous formulation optimization and stability testing to ensure reliable drug release and sustained efficacy.
Keywords
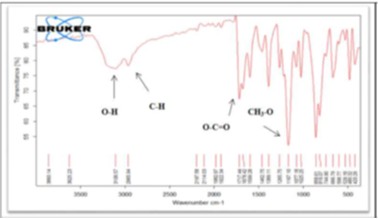
Naproxen Sodium Fast Dissolving Tablets, UV, HPLC, DSC, FTIR
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.