

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v19.3.narendra
Year: 2020, Volume: 19, Issue: 3, Pages: 1-6
Original Article
B Narendra Boggula1, M D Karvekar1,∗
1Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, Karnataka, India
*Corresponding Author
email: [email protected]
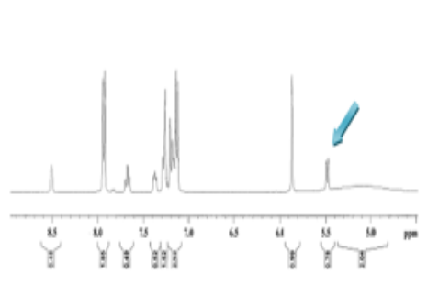
Racemic H1-antihistamine carbinoxamine contains enantiomers whose pharmacological characteristics may be different. The enantiomeric resolution of such molecules is important for maximising their efficacy and reducing side effects. The objective of this study was to achieve chiral resolution of racemic carbinoxamine maleate and evaluate the individual enantiomers based on their structural and pharmacological characteristics. Racemic carbinoxamine maleate was resolved by diastereomeric salt formation with O,O'-di-p-toluoyl-D-tartaric acid at R. L. Fine Chemicals, Bangalore. Crystallisation from isopropyl alcohol selectively afforded the (S)-isomer, and the (R)-isomer was obtained from the mother liquor. The free bases were recovered and transformed into maleate and oxalate salts, respectively. The analytical methods involved melting point determination, TLC, IR, optical rotation, and 1H NMR. Antihistaminic activity was tested using the guinea pig ileum at the Krupanidhi College of Pharmacy, Bangalore. The (S)-carbinoxamine base had an optical rotation of -2° and 95.83% inhibition of histamine, whereas the (R)-isomer had +4° rotation and an inhibition of just 9.09–12.5%. Different melting points and NMR singlets were used to verify enantiomeric purity. The IR spectra confirmed the differential interactions with the resolving agent. This study provides an effective resolution approach for carbinoxamine with a high enantiomeric yield and illustrates the higher pharmacological activity of the (S)-isomer. The findings of this study provide support for the development of enantiomerically pure formulations.
Keywords: Carbinoxamine, Chiral Resolution, Tartaric Acid, Antihistamine Activity
© 2020 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.