

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 4, Pages: 215–225
Original Article
Henerita Dash1,∗, Sudhir Kumar Sahoo2
1The Pharmaceutical College, Barpali, Odisha, India
2Royal College of Pharmacy and Health Sciences, Berhampur, Odisha, India
*Corresponding Author
Email: heneritadas31@gmail.com
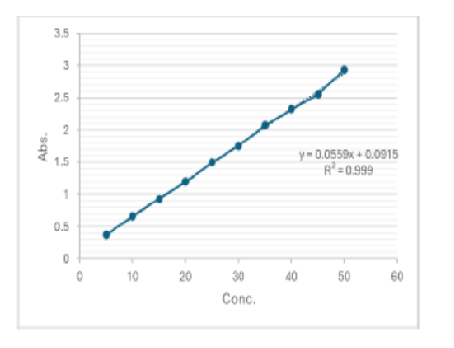
Background: The main goal of this experiment was to create and test a reliable and fast HPLC method for measuring the amount of Diltiazem, a drug that lowers blood pressure, in large quantities and to conduct stability studies. Methods: For the method development we use column of octa decyl silane which was bonded to porous silica with particle size 5µ ODS C-18 (250×4.6mm, 5µm), methanol and water mixture v/v proportion (90:10) used as the mobile phase, a sample of 25 µL was inlet into the column and the outlet comes out at 1ml/min flowing capacity. The Peak of analyte was detected at a retention time of 2.037 min. Results: A perfect positive linear relationship between the concentration (10-70μg/mL) and correlation coefficient of 0.999 for Diltiazem was showed by the calibration curve of Diltiazem. This method had a LOD value of 1.276 and a LOQ value of 3.86 respectively. The absolute recovery was 96.52% for Diltiazem tablet. Conclusion: The assay was stability-indicating because the degradation product from stress studies does not affect the detection of diltiazem.
Keywords: Diltiazem, RP-HPLC, Methanol, Validation
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.