

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v21i1.ms21.78
Year: 2022, Volume: 21, Issue: 1, Pages: 13-22
Original Article
Nirmala Limarkar 1, Dipika Chavda ✉ 1, Vaishali Thakkar 1, Tejal Gandhi 2
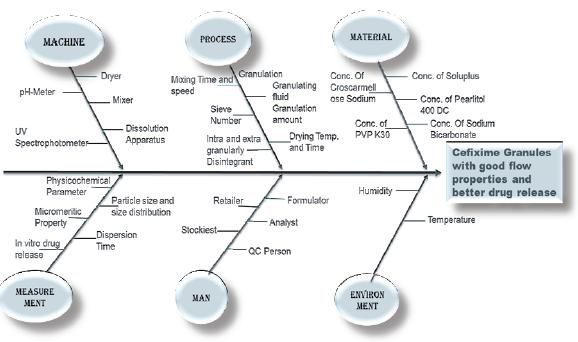
The present study was aimed to apply quality by design to develop stable and flexible pediatric granules of BCS class II drug Cefiximeto improve dissolution profile using Soluplus®. Thegranules were prepared by employing the wet granulation technique. The concentration of Soluplus®(X1), Croscarmellose sodium(X2), Sodium bicarbonate(X3) were identified as critical material attributes(CMA). The critical quality attribute (CQA) are percentage drug release in 20 minutes (Y1) and dispersion time (Y2). All 17 batches were evaluated for micromeritics properties, drug content, particle size distribution, dispersion time, granules' strength/friability, In vitro drug release, comparison with marketed product and stability study. All 17 batches from the Box Behnken showed all the test results values within limits, indicating that the prepared granules were of standard quality. The optimized batch of granules showed 94.45±0.26% drug release in 20min, while the dispersion time was 90.85±0.04 sec and the similarity factor (f2) was 56.39 to marketed product (ZIPRAX). In addition, the optimized batch exhibited no significant change in drug content, dispersion time, in vitro drug release after storage at 40°C ±2°C and 75% RH ± 5% RH for one month. The formulated granules showed better drug release and dispersion time. The granules can be reconstituted with water to prepare dispersion of the drug just before use to improve compliance of paediatric patients.
Keywords: Cefixime, granules, Quality by design, Box Behnken design, Soluplus®, Design space
© 2022 Published by Krupanidhi Educational Trust. This is an open-access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.