

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 2, Pages: 89-106
Original Article
Mohamed A Attia Shafie1,∗, Maha Abdel-Azeem Hassan Mohamed1, Ahmed Abd-El Zaher Saadi Mohamed1
1Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Assiut, 71526, Egypt
*Corresponding Author
Email: [email protected]
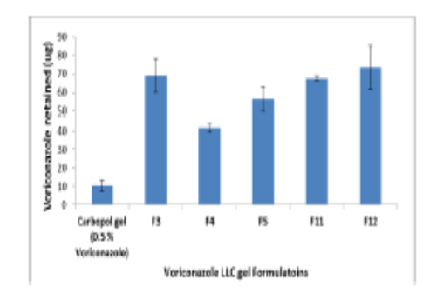
Topical antifungal therapy is recommended for the treatment of fungal infections of the skin. Due to its benefits, including the ability to direct drugs to the infection site and a lower chance of systemic adverse effects. The nano size and structure of LLC with the skin enhance the permeation and deposition of drugs within deep parts of skin. In this a novel formulation of voriconazole LLC gel was planned. Surfactant, co-surfactant, oil, and water pseudo-ternary phase diagrams were created in order to determine the LLC gel and microemulsion zones. The formulations were evaluated using polarizing microscopy, FT-IR spectroscopy. For each formulation, the polydispersity index, zeta potential, and mean droplet size were determined. The release patterns revealed that the prepared LLC gel provide sustained release profile up to 24 hr. Ex-vivo permeation of selected formulations exhibited higher permeability values for voriconazole. The drug skin deposition results from selected formulations showed that, in comparison to other studied formulations, a much larger amount of the drug was deposited in the skin after 24 hours. The confocal pictures appeared that the LLC gel was able to provide fluorescent color to all skin layers. The skin irritation study data of LLC gel formulations could be announced as safe and non- irritant for human skin. The selected formulations underwent a six-month stability testing at room temperature and 4–8 °C. The results showed that the pH, drug content, and viscosity values did not change significantly during this time.
Keywords: Lyotropic liquid crystal, Release and diffusion, Skin permeability, Skin deposition, Pathohistology
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.