

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 2, Pages: 107-110
Original Article
Alay Patel1,∗, Vidhi Thaker2, Vipul Chaudhari3
1Resident Doctor (3rd year), Department of Pharmacology, GCS Medical College, Hospital and Research Centre, Ahmadabad, Gujarat, India
2Assistant Professor, Department of Pharmacology, GCS Medical College, Hospital and Research Centre, Ahmadabad, Gujarat, India
3Professor, Department of Pharmacology, GCS Medical College, Hospital and Research Centre, Ahmadabad, Gujarat, India
*Corresponding Author
Email: [email protected]
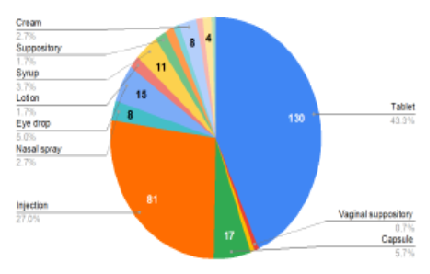
Medicine has advanced, necessitating access to accurate drug information. Package inserts (PIs) are crucial sources, approved by authorities, providing essential and updated drug details. Package inserts significantly impact patient compliance and drug effectiveness in chronic therapy. This study examines Indian market PIs, evaluating information quality and accessibility to improve medication safety. A prospective observational cross-sectional study was carried out. A total of 300 package inserts were collected from various pharmacies situated across different sites in Ahmedabad. Package inserts were scored out of 21 based on assessment criteria, expressed as percentages, and analysed descriptively. A total of 300 package inserts were analysed, among them 130 were tablets, 81 were injections, 17 were capsules, 15 were eye drops and the rest includes syrup, ear drops, nasal drops, suppositories, powder, ointment, gel, cream, lotion and suspension. 209 PIs were single drug preparations while 91 were fixed dose combinations. More than 90% of the score was achieved by 37 PIs. 96 PIs had scored between range 81% to 90%, while 86 PIs had scored between 71% to 80%. Only 6 PIs had scored below 50%. Most commonly missing information was the average duration of treatment (83% of PIs), excipients (93% of PIs), and shelf life (81% of PIs). The finding of this study revealed that, although only 2% of PIs had scored below 50%, some crucial information was lacking from a major number of PIs. Regular review and collaboration among stakeholders ensure updated, reliable and comprehensive information, benefiting patient care and healthcare delivery.
Keywords: Drug information, Package insert, Indian market
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.