

Journal of Pharmaceutical Research
Year: 2023, Volume: 22, Issue: 4, Pages: 178-185
Review Article
Shaik Ayesha Ameen1,*, Nagaraju Pappula2
1 Research scholar, Hindu college of pharmacy, Guntur, Andhra Pradesh
2 Professor and HOD, Department of pharmaceutical analysis, Hindu college of pharmacy, Guntur, Andhra Pradesh
*Corresponding author email: [email protected]
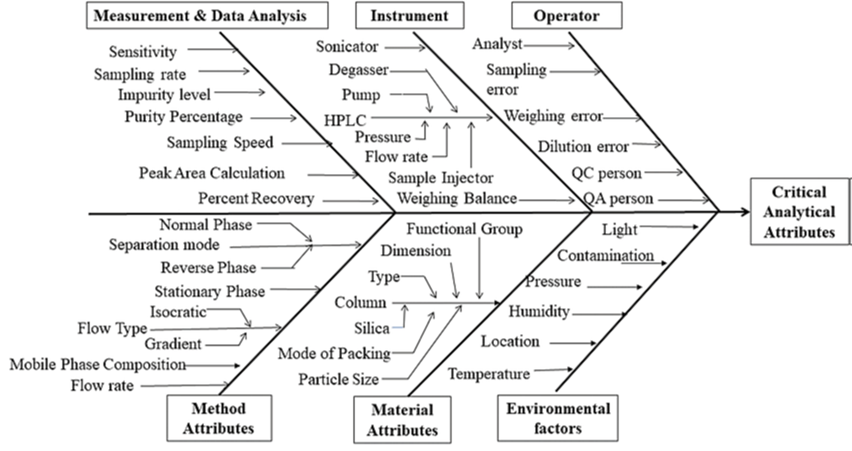
As per the present scenario traditional techniques of quality by testing of finished pharmaceutical products have proved to be unsatisfactory, although focus on 'Total quality assurance can be attained only by in-process testing and analysis. The Quality by Design (QbD) idea has already been established and applied in all nations that adhere to the International Conference on Harmonization Guidelines. The proposed ICH Guidelines Q8 for pharmaceutical development, Q9 for quality risk management, and Q10 for pharmaceutical quality systems offer a foundation for incorporating quality into the product. Other components are being added and integrated into the analytical method development process, including as Quality Risk Management, the Pharmaceutical Quality System, and Process Analytical Technology (PAT) guidelines. The Quality by Design (QbD) idea has already been established and applied in all nations that adhere to the International Conference on Harmonization Guidelines. The proposed ICH Guidelines Q8 for pharmaceutical development, Q9 for quality risk management, and Q10 for pharmaceutical quality systems offer a foundation for incorporating quality into the product. Other components are being added and integrated into the analytical method development process, including as Quality Risk Management, the Pharmaceutical Quality System, and Process Analytical Technology (PAT) guidelines. They are widely acknowledged in the business as AQbD (Analytical Quality by Design) ideas. This paper attempts to summarize QbD achievements to date, regulatory perspectives on QbD, a comparative examination of conventional and analytical QbD techniques in method development, and issues about its fundamental application.
Keywords
Quality by Design, Design of Experiments, Analytical Quality, Critical Quality Attributes Analytical Target Profile, Process Control
© 2023 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.