

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v24.i4.108
Year: 2025, Volume: 24, Issue: 4, Pages: 195-205
Original Article
Nikita Ghosh1, Rajat Das1*, Jyochhana Priya Mohanty1, Pallab Ghosh1, Chandrika Sharma1, Shreetama Roy1
1Department of Pharmacognosy, Himalayan Pharmacy Institute, Majhitar, East Sikkim-737136, India
*Corresponding author.
Rajat Das
Email: [email protected]
Stephania japonica plants appear to be rich in secondary metabolites, widely utilised in traditional medicine to treat and heal numerous illnesses. It is traditionally used to treat wounds, but there is no scientific data on its in vivo wound-healing activity. Pharmacognostical analysis evaluates a drug's identity, quality, purity, and safety through microscopic and macroscopic research. The extraction solvent dissolves significant amounts of the target material. The percentage yields of alcohol and water-soluble extractive values were calculated. After the extraction of the plant material, the herbal ointment was formulated. After checking the evaluation parameter of the ointment, the excision wound healing activity was performed on Wistar rats. The herbal extract was prepared by using a simple Soxhlet extraction process to obtain a good yield of extract, and there was no harm to the chemical constituents and their activity. This study showed that the herbal ointment heals wounds better in less time as similar with the standard ointment by applied to Wistar rats.
Keywords: Pharmacognostical, Phytochemical, Wound healing, <i>Stephania japonica</i>, Herbal ointment
The family Menispermaceae, which has 350 species and 65 genera, includes the genus Stephania. This family is widely dispersed throughout the tropics. This family primarily consists of plants and shrubs, with very few trees. There are thin climbers with peltate and membrane leaves in the Stephania genus. Axillary inflorescences with umbelliform blooms develop from old, leafless stalks. Traditional uses of these herbs include the treatment of asthma, TB, dysentery, hyperglycemia, cancer, fever, digestive problems, sleep disorders, and inflammation [1]. Stephania japonica is a climbing plant that develops from a woody rhizome to generate thin stems that may eventually begin to resemble wood. The flavour of the tuberous root is astringent and unpleasant. It is used to treat hepatitis, fevers, stomach aches and dyspepsia, diarrhoea and dysentery, and urinary tract infections. It is claimed that the root might help treat itchiness because it contains picrotoxin. Discolour is bitter and extremely dangerous. Fever, diarrhoea, illnesses of the urinary system, and stomachaches can all be treated with it medicinally. Breast infections are treated with a mixture of crushed leaves and water that has a little gelatinous consistency [2]. Anti-oxidant, analgesic, antinociceptive, anti-microbial, anti-inflammatory, anti-hyperglycemic, anti-hyperlipidemic, neuroprotective, and antidiarrheal activities of the plant of Stephania japonica are already documented by various scientific committees [3-9]. Although it has historically been used to treat wounds, there is no scientific evidence about its ability to cure wounds in-vivo. This study focuses on the identification, quality, purity, and safety of drugs for human use, specifically evaluating the physicochemical characteristics of the Stephania japonica plant. The herbal extract was prepared by using a simple Soxhlet extraction process with petroleum ether, benzene, acetone, ethyl acetate, methanol and ethanol according to their polarity. This study showed that the herbal ointment which is prepared from the ethanolic extract heals wounds better in less time compared with the standard ointment by applied to Wistar rats.

Plant materials: Stephania japonica
Selection & collection: Stephania japonica's stems were selected based on traditional uses and a literature survey [10]. The plant parts were collected from Rangpo forest, Sikkim.
Identification & authentication: The plant was identified through standard literature and authenticated by BSI, Gangtok, with accession number SHRC-5/02/2022-23/tech-200.
After collecting, the plant parts were washed thoroughly with tap water to remove any unwanted materials present on them. This was further dried in the shade for a few days. After complete drying, dried plant materials were coarsely powdered with the help of a mechanical grinder and passed through a sieve and stored in a tightly closed container.
Methanol (finar), ethyl acetate (finar), hydrochloric acid, potassium bismuth iodide, potassium mercuric iodide, alcoholic alpha naphthol, sulphuric acid, Fehling’s reagent A & B (oxford lab fine chem llp), glacial acetic acid (sdfcl), ferric chloride (sdfcl), acetic anhydride, chloroform (finar), gelatin, sodium chloride, ethanol (finar), acetone (finar), petroleum ether (finar), benzene, iodine, hydrochloric acid, benedict reagent (dey’s chemical works), magnesium ribbon, sodium hydroxide, metallic zinc (finar), ammonia.
Digital balance, heating mantle (sunsim), hot air oven, rotary evaporator (hahnvapor), water bath, Soxhlet apparatus.
Beaker, measuring cylinder, funnel, glass rod, petri-dish, china-dish, conical flask, round-bottom flask, glass slide, test-tube, test-tube holder, test tube stand, spatula, etc.
Macroscopic examination:
The macroscopical observation was carried out according to the standard methods to determine the shape, size, colour, taste and odour.
Powder microscopy:
After dissolving powdered plant material in distilled water, the drug's minute particles were dipped in colouring agents such as rheuthenium red, quick green, Safranine, Sudan IV, and iodine, cleaned, mounted, and examined under an electron microscope. The microscopic characteristics were recorded.
Total ash:
A tar-coated platinum or silica dish containing precisely two grams of air-dried medicament was ignited at a temperature of not more than 800 °C until the substance was carbon-free [11]. After cooling, the weight was recorded. If a carbon-free ash could not be obtained, the burned mass was cleansed with hot water, and the residue was gathered. On ashless filter paper, the residue was burned together with the filter paper until the ash was white or almost white. Once the filtrate had totally evaporated, it was put in the dish. It was calculated as a proportion of the overall ash content of the medication.
Acid-insoluble ash:
Using 25 millilitres of 2M HCL acid to boil the ash (from total ash) for five minutes, the insoluble debris was collected in an Ashless Filter Paper or Gooch Crucible. Following a hot water cleaning, it was ignited, allowed to cool in a desiccator, and then weighed. The percentage of acid-insoluble ash was calculated using the drug base that was dried.
Water-soluble ash:
The ash (total ash technique) was boiled for five minutes with 25 cc of water, and the insoluble materials were collected in a Gooch crucible or on ashless filter paper. Following a fifteen-minute burn at a temperature not to exceed 800 °C, the mixture was washed with hot water. Water-soluble ash displays the weight differential following the deduction of the insoluble material's weight from the ash's weight. Next, using dry medications as a base, the percentage of water-soluble ash was computed [12].
Moisture content:
The loss-on-drying method, which involves heating a substance at 105℃ for 4 hours and weighing. Again, heat for 30 mins and weigh again. Repeat this process until the weight of plant material is constant, and calculate the percentage of weight loss after complete drying using the following formula:
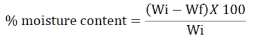
Where Wi = sample initial weight.
Wf = sample weight after drying.
Extractive value:
The extracts obtained by exhausting crude drugs are indicative of approximate measures of their chemical constituents. Taking into consideration the diversity in chemical nature and properties of drug contents, alcohol and water were used for the determination of extractive values.
Preparation of plant extract:
Stephania japonica was extracted using a Soxhlet apparatus. 35 g of the powdered plant material was packed in a thimble and extracted using 300 mL of solvent. The plant material was extracted using a successive solvent extraction process with petroleum ether, benzene, acetone, ethyl acetate, methanol and ethanol. After completion of the extraction, concentrated the extract was concentrated using a rotary evaporator and a water bath.
Phytochemical screening of Stephania japonica:
Different extracts of S. japonica were evaluated phytochemically as per standard protocol to find out the presence of different phyto-constituents [13].
Thin-layer chromatography:
TLC is a crucial separation technique in plant chemistry, aiding in the identification of known and unknown compounds. Pre-coated silica plate was used as the stationary phase, and ethyl acetate: acetic acid: formic acid: water (100:11:11:27) was used as the mobile phase for the Flavonoid identification using Rutin as a standard compound.
Ointment formulation:
Based on higher extractive value and results of phytochemical analysis, the ethanolic extract was used for ointment formulation. The ointment was formulated according to the British Pharmacopoeia using the following ingredients [14]:
| Ingredients | Quantity (gm) |
|---|---|
| White soft paraffin | 85 |
| Cetostearyl alcohol | 4.5 |
| Wool fat | 2.5 |
| Hard paraffin | 2.5 |
| Plant extract | 5 |
| Methyl paraben | 0.5 |
| Lavender oil | 1-2 drops |
| Total | 100 |
Physical evaluation: Physical parameters like colour and odour were examined by visual examination.
Viscosity: The viscosity of the formulated ointment was measured using a Brookfield Viscometer.
pH: The pH of the prepared herbal ointment was determined in triplicate using a digital pH meter, and the average value was calculated.
Spreadability: Spreadability was assessed by placing an extra sample within two glass slides, then compressing it to a consistent thickness with a specific weight for a predetermined amount of time. The measure of spreadability was the amount of time needed to separate the two slides. Improved spreadability is the outcome of separating two slides in less time. The formula below was used to calculate spreadability.
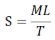
Where, S= Spreadability, M= Weight tide to the upper slide, L= Length of glass slide, T= Time taken to separate the slides.
Centrifugation: For assessing accelerated ointment deterioration, it is thought to be a special tool. By centrifuging at 10,000 rpm for 10 minutes, it was ascertained.
Washability: Formulation was applied on the skin, and then the ease of removal of water was checked.
Loss on drying: The sample's weight loss is primarily due to the presence of water, which was determined.
Acute Skin Irritation Test: The formulated ointment was applied topically to Human skin, and the results were noted down.
For this investigation, Wistar albino rats weighing between 180 and 200 g were employed. Animals were purchased from the Himalayan Pharmacy Institute's Majhitar authorised animal house after approval from the Institutional Animal Ethical Committee with the approval number HPI/2023/60/IAEC/PP-0208. The rats were placed into 3 groups, and each group had six rats (n=6). Group 1 referred to the disease control group, where no treatment was done; group 2 was referred to as the standard group, which was treated with povidone-iodine ointment [15] and group 3 was treated with ointment prepared from Stephania japonica extract [16]. In a cross-ventilated animal housing with a temperature of 25°C, a relative humidity of 44-56%, and a light-to-dark cycle of 12:12 hours, the animals were acclimated to the typical laboratory settings. They were then fed regular food and given access to water whenever needed throughout the study. According to the technique outlined below, excision wounds were made. Six rats (n = 6) from each of three groups of animals will have their dorsums shaved before being given 50 mg/kg (i.p.) of ketamine hydrochloride to put them to sleep. An impression was created on the shaved dorsal region, and the location of the wound was indicated. A 2.5 cm long full-thickness excision wound was made along the marking with toothed forceps, a surgical blade, along sharp scissors. Rats were released into their natural environment unclothed. From the day of the procedure until complete healing, the simple ointment bases, designed extract ointment, and standard drug were used once daily [17, 18].
Percentage wound healing = (healed area/total wound area) x 100.
| Shape | Elongated |
|---|---|
| Taste | Spicy flavor |
| Odour | Indistinct |
| Colour | Brownish green |
| S. No | Parameters | Values (in percentages) |
|---|---|---|
| 1. | Total ash % w/w | 7.75 |
| 2. | Acid insoluble ash %w/w | 2.35 |
| 3. | Water soluble ash %w/w | 5.15 |
Extractive Value of Stephania japonica:
| Sl. No | Types of solvent | Colour | Extractive value in percentage (w/w) |
|---|---|---|---|
| 1 | Alcohol soluble extractive value | Reddish brown | 7.94% |
| 2 | Water-soluble extractive value | Brown | 4.12% |
| Phyto-constituent | Pet. ether extract | Benzene extract |
Acetone extract |
Ethyl acetate extract |
Methanol extract |
Ethanol extract |
|---|---|---|---|---|---|---|
| Alkaloid | - | + | + | + | + | + |
| Carbohydrates | + | + | + | + | + | + |
| Saponins | - | - | - | - | - | - |
| Glycosides | - | - | - | - | - | - |
| Tannins | - | - | - | + | - | - |
| Triterpenoid & Steroid | + | + | + | + | + | + |
| Flavonoids | - | - | - | - | + | + |
| Phenols | - | + | + | + | + | + |
‘+’ indicates present and ‘-’ indicates absent
Thin Layer Chromatography: TLC of the ethanolic plant extract was performed using Rutin as a standard compound.
| Sl. No. | TLC study | Solvent system | Rf |
|---|---|---|---|
| 1 | Ethanolic plant extract | Ethyl acetate: acetic acid: formic acid: water (100:11:11:27) | 0.42 |
| 2 | Rutin | Ethyl acetate: acetic acid: formic acid: water (100:11:11:27) | 0.44 |
| Ingredients | Master Formula (g) | Reduced Formula (g) |
|---|---|---|
| White soft paraffin | 850 | 85 |
| Cetostearyl alcohol | 45 | 4.5 |
| Hard paraffin | 25 | 2.5 |
| Wool fat | 25 | 2.5 |
| Plant extract | 50 | 5 |
| Methyl paraben | 5 | 0.5 |
| Lavender oil | 2-3 drops | 1-2drops |
| Total | 1000 | 100 |

| Physiochemical Parameter | Observation |
|---|---|
| Colour | Pearl white |
| Odour | Characteristic |
| Viscosity | Cp 5303 |
| pH | 5.5 |
| Spreadability | 0.334 g.cm/sec |
| Washability | Good |
| Centrifugation | No phase separation |
| Loss on drying | 8.23 |
| Acute skin irritation | No irritation |
The current research will utilise healthy Wistar albino rats weighing between 180 and 200 g. Animals must be obtained from the Himalayan Pharmacy Institute's licensed animal shelter in Majhitar, Sikkim, India. The rats were divided into three groups, with six rats in each group.
| Day | Date | Mean Wound length(cm) | Percentage Wound Healing | Picture |
|---|---|---|---|---|
| Control group: | ||||
| 1st | 20.05.2023 | 2.5±0.058 | - |  |
| 3rd | 23.05.2023 | 2.2±0.060 | 12 |  |
| 6th | 26.05.2023 | 1.8±0.052 | 28 | 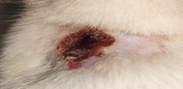 |
| 9th | 29.05.2023 | 1.6±0.037 | 36 |  |
| 12th | 01.06.2023 | 1.3±0.037 | 48 | 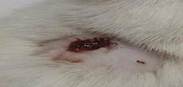 |
| 15th | 04.06.2023 | 1.1±0.037 | 56 | 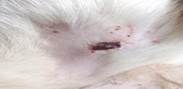 |
| 18th | 07.06.2023 | 1±0.075 | 60 | 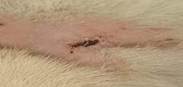 |
| 21st | 10.06.2023 | 0.8±0.089 | 68 | 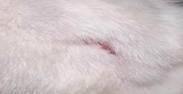 |
|
|
||||
| 1st | 20.05.2023 | 2.5±0.036 | - | 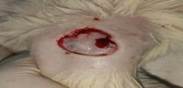 |
| 3rd | 23.05.2023 | 2.0±0.048 | 20 | 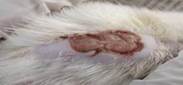 |
| 6th | 26.05.2023 | 1.5±0.027 | 40 | 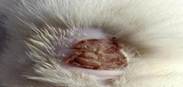 |
| 9th | 29.05.2023 | 1.3±0.025 | 48 |  |
| 12th | 01.06.2023 | 1.2±0.038 | 52 |  |
| 15th | 04.06.2023 | 1.0±0.012 | 60 |  |
| 18th | 07.06.2023 | 0.8±0.02 | 68 | 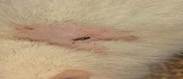 |
| 21st | 10.06.2023 | 0.6±0.009 | 76 |  |
|
|
||||
| 1st | 20.05.2023 | 2.5±0.039 | - | 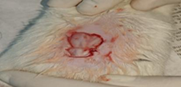 |
| 3rd | 23.05.2023 | 1.6±0.025 | 36 | 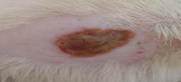 |
| 6th | 26.05.2023 | 1.4±0.030 | 44 | 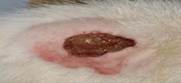 |
| 9th | 29.05.2023 | 1.3±0.029 | 48 |  |
| 12th | 01.06.2023 | 1.1±0.018 | 56 | 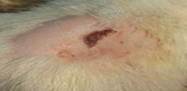 |
| 15th | 04.06.2023 | 0.8±0.017 | 68 | 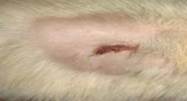 |
|
18th |
07.06.2023 |
0.4±0.007 |
84 |
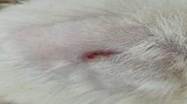 |
| 21st | 10.06.2023 | 0.1±0.015 | 96 | 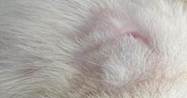 |
The excision wound is set to be formed using the procedure described below. Three groups of six rats each will be trimmed on the dorsum and sedated with ketamine hydrochloride (50 mg per kg, i.p., weight of body weight). After shaving the dorsal region, an impression must be created, and the place where the wound will be made must be indicated. The wound area was measured on 3th, 6th, 9th, 12th, 15th, 18th, and 21st day compared with the wound area on the first day. The produced extract ointment and normal medication should be used once a day from the beginning of the procedure. Using the following formula, the degree of wound recovery was determined as the percentage closure in the wound area relative to the initial wound area:
Percentage wound contraction = 100-[Final diameter (cm) x 100/ initial diameter (cm)]
Wound area was measured in individual animals, and mean wound length was calculated for each group.
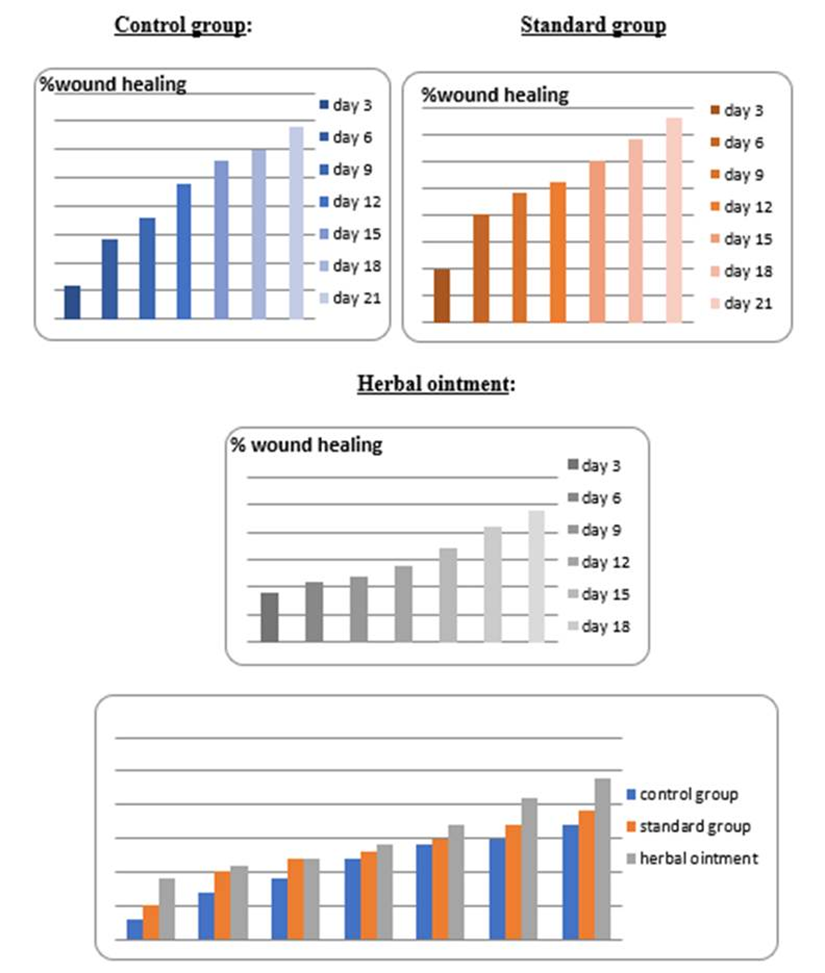
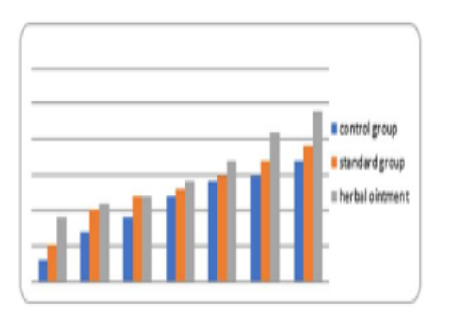
As a result of this search, these were what emerged as the most interesting parts of previous works focused on research: evaluating antioxidant, anti-nociceptive [4, 19, 20] and anti-inflammatory" properties [21] or cytotoxicity potential derived from leaves extract allocation type [22] of Stephania japonica instead of concentrating on wound application effects [23]. Researchers have concluded that enough steroids, alkaloids, carbohydrate phenols and flavonoids exist in Stephania japonica extracts after phytochemical screening. An herbal balm was formulated using the Stephania japonica extracts, and its wound-healing activity was assessed in an animal (Wistar rat) excision wound model. The results of the study revealed that the herbal ointment belonging to the Stephania Japonica group possessed wound healing potential. From day 0 to the end of the study, wound healing was observed and recorded, in which the herbal ointment group showed superior progress than that of the control group ([Fig. 1]) [24]. The findings of the present study, taken together, suggest that the wound healing potential of Stephania japonica is promising when formulated as an ointment. The plant owes its wound healing properties to its immense number of phytochemical constituents [25]. For proper application in managing injuries, including pressure ulcers, more research is needed to understand the body's wound healing mechanisms and thoroughly optimise the ointment formulation [26].
The following are some of the most important findings from the most recent research on the use of Stephania japonica extract in wound healing ointments:
The scope of the inquiry: Past examinations zeroed in on assessing the cancer prevention agent, pain-relieving, and cytotoxic properties of Stephania japonica leaf extricates, without directly exploring wound recuperating applications. Current research made an herbal ointment from Stephania japonica and tested how well it healed wounds in an animal model.
Phytochemical investigation: Steroids, alkaloids, carbohydrates, phenols, and flavonoids were found in Stephania japonica extracts in previous research. Flow research based upon the phytochemical profile to foster an injury recuperating balm definition [27].
Evaluation of wound healing: Previous studies did not evaluate the ability of Stephania japonica extracts to heal wounds. Current research developed an in vivo excision wound model using Wistar rats to demonstrate the effectiveness of the Stephania japonica-based ointment for wound healing [28].
Outcomes: Stephania japonica extracts were found to have antioxidant, analgesic, and cytotoxic properties in previous research [29]. Momentum research showed that the Stephania japonica-based treatment fundamentally worked on injury healing in the animal model, with the injury healing notice and recorded for more than 21 days [30, 31].
These studies help to ensure the identification, quality, purity, and safety of the drug for human use. The physicochemical characteristics of the Stephania japonica plant have been attempted to be evaluated. It is critical to identify plant material both taxonomically and pharmacognostically in order to establish pharmacognostical standards and prevent the use of fake and adulterated medications. From West Bengal, the plant is collected. The plants used in this study were chosen based on how well they work in traditional medical systems. The extractive value was afterwards calculated using a Soxhlet extraction. The goal of this study was to create and assess an herbal ointment. To achieve a good yield of extract and avoid harming the chemical contents and their activity, the herbal extracts were made using a straightforward Soxhlet extraction procedure. Then the simple ointment base was formulated and kept in a beaker for further formulation of the herbal ointment. The phytochemical analysis of extracts of Stephania japonica stem revealed the presence of Steroids, alkaloids, Carbohydrates, phenols, flavonoids. After the extraction of the plant material the herbal ointment was formulated. The herbal ointment evaluation parameter was performed. After checking the evaluation parameter, the wound healing activity was performed on wistar rats. The animals are divided into three groups. Group-I control group, group-II standard group and group-III herbal ointment. From the zero day it started to be observed, then at 3rd, 6th, 9th, 12th, 15th, 18th and 21st day it was observed and results found. From this experiment of wound healing activity of excision model, it was observed that the herbal ointment have the good healing properties and heals the wound similar to the standard drug, so this can be useful for the further use in wound healing activity.
FDA; Food and Drug Administration, TM; Traditional Medicine, BSI; Botanical Survey of India, Cp; Centipoise, IL; Interleukin, VEGE; Vascular Endothelial Growth Factor, FE; Iron, BHA; Beta Hydroxy Acid, DPPH; 2,2-diphenylpicrylhydrazyl, ABTC; 2,2'- azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), H2SO4; Sulfuric Acid, ml; Milliliter, nm; Nanometer, NaOH; Sodium hydroxide, mg; Milligram
1. Gaur RD. Flora of the District Garhwal, Northwest Himalaya. Transmedia; 1999.
2. Fern K. Useful Tropical Plants Database, 2023. http://tropical.theferns.info/.
3. Uddin SN, Amin MN, Shahid-Ud-Daula AF, Hossain H, Haque MM, Rahman MS, <I>et al</I>. Phytochemical screening and study of antioxidant and analgesic potentials of ethanolic extract of <I>Stephania japonica</I> Linn. Journal of Medicinal Plants Research. 2014; 8 (37). Available from: https://doi.org/10.5897/jmpr2014.5443
4. Moniruzzaman M, Hossain MS, Bhattacharjee PS. Evaluation of antinociceptive activity of methanolic extract of leaves of <I>Stephania japonica</I> Linn. Journal of Ethnopharmacology. 2016; 186 Available from: https://doi.org/10.1016/j.jep.2016.04.008
5. Minhajur RM, Alam MM, Daula AS, Shahriar M, Moghal MM, Siddiqui R. The antimicrobial activity and brine shrimp lethality bioassay of leaf extracts of <I>Stephania japonica</I> (akanadi). Bangladesh Journal of Microbiology. 2012; 28 (2). Available from: https://doi.org/10.3329/bjm.v28i2.11816
6. Ahmed NU, Akter R, Satter MA, Khan MS, Islam F, Abdullah AM. Anti-inflammatory, antioxidant and anti-diarrheal effects of ethanol extract of <I>Stephania japonica</I>. Bangladesh Journal of Scientific and Industrial Research. 1970; 46 (4). Available from: https://doi.org/10.3329/bjsir.v46i4.9587
7. Sultana MC, Alam MB, Asadujjaman M, Akter MA. Antihyperglycemic and antihyperlipidemic effects of <I>Stephania japonica</I> (Thunb.) Miers. Tenril in alloxan-induced diabetic mice. International Journal of Pharmaceutical Sciences and Research. 2012; 3 (8). Available from: http://dx.doi.org/10.13040/IJPSR.0975-8232.3(8).2726-32
8. Xiao J, Hao T, Chen G, Song J, Lin B, Li W, <I>et al</I>. Natural neuroprotective alkaloids from <I>Stephania japonica</I> (Thunb.) Miers. Bioorganic Chemistry. 2019; 91 Available from: https://doi.org/10.1016/j.bioorg.2019.103175
9. Yiblet TG, Tsegaw A, Ahmed N, Dagnew SB, Tadesse TY, Kifle ZD. Evaluation of Wound Healing Activity of 80% Methanol Root Crude Extract and Solvent Fractions of <I>Stephania abyssinica</I> (Dill. & A. Rich.) Walp. (Menispermaceae) in Mice. Journal of Experimental Pharmacology. 2022; 31 Available from: https://doi.org/10.2147/jep.s364282
10. Applequist WL, Miller JS. Selection and authentication of botanical materials for the development of analytical methods. Analytical and Bioanalytical Chemistry. 2013; 405 (13). Available from: https://doi.org/10.1007/s00216-012-6595-1
11. Yadav M, Chatterji S, Gupta SK, Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. <I>International Journal of Pharmacy and Pharmaceutical Sciences</I>. 2014;6(5):539- 542.
12. Smith G. Herbs in Medicine. <I>Practice</I>. 2004;154:439-41.
13. Khandelwal KR, Sethi V. Practical Pharmacognosy. 26th Edition. Nirali Prakashan, India. 2016; 25.1-25.9.
14. Carter SJ. Cooper and Gunn’s Dispensing for Pharmaceutical Students; CBS Publisher and Distributors: Delhi, India, 1997; pp. 199–200.
15. Goldenheim PD. An appraisal of povidone-iodine and wound healing. <I>Postgraduate Medical Journal</I>. 1993;69 Suppl 3:S97-105.
16. Nagajothy S, Vijayabaskaran M, Sambath Kumar R, Balasubramanian P, Gomathi G, Kannan C. Evaluation of antiasthmatic activity of <I>Stephania japonica</I> Thunb. Miers. <I>International Journal of Chemical and Pharmaceutical Sciences</I>. 2015;6(1):70-6.
17. Nagar HK, Srivastava AK, Srivastava R, Kurmi ML, Chandel HS, Ranawat MS. Pharmacological investigation of the wound healing activity of <I>Cestrum nocturnum</I> (L.) ointment in Wistar albino rats. Journal of Pharmaceutics. 2016; 2016 Available from: https://doi.org/10.1155/2016/9249040
18. Chen RF, Wang CT, Chen YH, Chien CM, Lin SD, Lai CS,<I>et al</I>. Hyaluronic Acid–Povidone-Iodine Compound Facilitates Diabetic Wound Healing in a Streptozotocin-Induced Diabetes Rodent Model. Plastic & Reconstructive Surgery. 2019; 143 (5). Available from: https://doi.org/10.1097/prs.0000000000005504
19. Al-Amin MY, Lahiry A, Ferdous R, Hasan MK, Kader MA, Alam AK, <I>et al</I>. <I>Stephania japonica</I> Ameliorates Scopolamine‐Induced Memory Impairment in Mice through Inhibition of Acetylcholinesterase and Oxidative Stress. Advances in Pharmacological and Pharmaceutical Sciences. 2022; 2022 (1). Available from: https://doi.org/10.1155/2022/8305271
20. Macek D, Holthusen H, Rjosk A, Ritzert S, Lautenschläger T, Neinhuis C, <I>et al</I>. Mechanical investigations of the peltate leaf of <I>Stephania japonica</I> (Menispermaceae): Experiments and a continuum mechanical material model. Frontiers in Plant Science. 2023; 13 Available from: https://doi.org/10.3389/fpls.2022.994320
21. Udegbunam SO, Igbokwe NP, Udegbunam RI, Nnaji TO, Anyanwu MU. Evaluation of wound healing and antibacterial properties of methanolic root extract of <I>Stephania dinklagei</I> (Engl.) Diels. African Journal of Traditional, Complementary and Alternative Medicines. 2015; 12 (6). Available from: https://doi.org/10.21010/ajtcam.v12i6.12
22. Ahangar N, Jouybari HB, Davoodi A, Shahani S. Phytochemical Screening and Antinociceptive Activity of the Hydroalcoholic Extract of <I>Potentilla reptans</I> L. Pharmaceutical and Biomedical Research. 2022; Available from: https://doi.org/10.18502/pbr.v7i4.9375
23. Akter N. Antibacterial Sensitivity Test of Crude Extract of (<I>Curcuma zedoaria, Solanum virginianumand, Stephania japonica</I>) & Resistant Pattern of Clinically Isolated Bacteria against Conventionally Used Antibiotics (Doctoral dissertation, East West University).
24. Zehad A, Islam GJ, Rashid M, Juthy NJ, Zannah S. Antidiabetic and antihyperlipidemic activities of methanolic leaf extract of <I>Stephania japonica</I> in Alloxan Induced Diabetic Rats. Pharmacology & Pharmacy. 2017; 08 (04). Available from: https://doi.org/10.4236/pp.2017.84008
25. Das AK, Molla S, Sykat MR, Ali A, Haque T, Rahman L, <I>et al</I>. Phytochemical and pharmacological review on <I>Stephania japonica</I>. International Journal of Pharma Sciences and Research. 2019; 3 (6). Available from: https://doi.org/10.26717/bjstr.2019.14.002500
26. Islam MT, Hossain M, Azad MA, Tareq SM. Botanical and phyto-pharmacological reports on <I>Stephania japhonica </I>. International journal of applied pharmaceutical sciences and research. 2017; 2 (01). Available from: https://doi.org/10.21477/ijapsr.v2i1.6980
27. Islam MT, Khatun MJ. Antioxidant-mediated neuro-protective and GABAergic calming effect of <I>Stephania japhonica </I>. International Journal of Medicine. 2017; 5 (2). Available from: https://doi.org/10.14419/ijm.v5i2.7932
28. Bokshi B, Rahman SA, Sadhu SK, Muhammad A, Islam MA. Assessment of analgesic and antidiarrhoeal activities of different fractions of crude extract of <I>Stephania japonica</I> stem. International journal of pharmaceutical sciences and research. 2013; 4 (3). Available from: https://doi.org/10.13040/ijpsr.0975-8232.4(3).1013-21
29. Ahmed MF. Evaluation of antioxidant activity of <I>Stephania Japonica</I> and <I>Mikania cordata</I> (Doctoral dissertation, East West University).
30. Shankar R, Neyaz S, Anku G, Rungsung W. Exploration, Conservation, and Cultivation of <I>Stephania japonica</I> (Thunb.) Miers. Journal of Drug Research in Ayurvedic Sciences. 2020; 5 (3). Available from: https://doi.org/10.5005/jdras-10059-0098
31. Islam MD, Islam A, Tasnin N, Akter SF, Uddin MS. Exploration of antioxidant and anticancer activity of <I>Stephania japonica</I> leaves extract. Journal of Pharmaceutical Research International. 2021; 33 (28A). Available from: https://doi.org/10.9734/jpri/2021/v33i28a31529
© 2025 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.