

Journal of Pharmaceutical Research
Year: 2025, Volume: 24, Issue: 4, Pages: 216-223
Original Article
Vothani Sarath Babu1*, Chakali Manoj Kumar1, P Sumalatha1, Pallavi Chittoor2, OVS Reddy2
1Department of Pharmaceutics, SVU College of Pharmaceutical Sciences, Sri Venkateswara University, Tirupati 517502, AP, India
2Department of Biochemistry, S V U College of Sciences, Sri Venkateswara University, Tirupati 517502, AP, India
*Corresponding author
Vothani Sarath Babu
Email: [email protected]
The Aim of this present study was to develop an oral sustained release matrix tablet of metformin HCl and evaluate the influence of Xanthum gum on the drug release profile. The use of FTIR for drug – excipient compatibility is appropriate. The wet granulation method is suitable for matrix tablet preparation, incorporating hydrophilic HPMC K100M, PVP K30, and Xanthan gum as controlling agents for the release rate. Lactose served as the filler. The physicochemical tests (weight variation, hardness, friability, content uniformity, and in vitro dissolution) were standard and appropriately listed. Both pre- and post-compression parameters are good that both stages were evaluated in this study. The data on drug release were analyzed using multiple models to determine the kinetics and mechanism of drug release. A clear comparison between HPMC alone and HPMC – Xanthum gum combination was made. Quantify the difference in drug release, HPMC alone released 90% of drug within 6 hours, while the combination extended release to 12 hours. Release kinetics following Zero order kinetics with Higuchi model diffusion-controlled release mechanism. Formulation F8 was identified as the optimal formulation. The dissolution profile of formulation F8 can be characterized by Release kinetics following Zero order kinetics with Higuchi model diffusion-controlled release mechanism.
Keywords: Matrix tablets, Metformin hydrochloride, Xantham gum, HPMCK100, PVPK30
Diabetes is a significant contributor to death and disability around the globe. It is a health condition that hinders the body's ability to manage blood glucose levels [1]. Diabetes mellitus is a complex metabolic disorder characterized by elevated blood sugar levels due to inadequate insulin production, ineffective insulin action, or a combination of both [2]. There are two main classifications of diabetes. The first type is type I, also referred to as juvenile diabetes or insulin-dependent diabetes, while the second type is type II, known as non-insulin dependent diabetes mellitus. Type II diabetes is the most prevalent form of diabetes, as noted by the National Institute of Diabetes. Typically, type II diabetes mellitus develops in adulthood and predominantly affects older and obese individuals. Metformin hydrochloride is a primary biguanide hypoglycemic agent utilized to manage non-insulin-dependent diabetes mellitus that does not respond to dietary changes. It does not lead to lactic acidosis. Metformin enhances glucose tolerance by lowering both fasting and post-meal glucose levels through a reduction in intestinal glucose absorption, liver glucose production, fat synthesis, and glucose uptake by fat and muscle cells [3]. It is included in the World Health Organization's list of essential medications, which comprises the most effective and safe drugs necessary for a healthy system. The oral bioavailability of metformin ranges from 50-60%, and its average elimination half-life is 6.2 hours, which requires frequent high-dose administrations to maintain adequate plasma concentrations, thereby reducing patient compliance and heightening the risk of negative side effects [4-7]. Various studies have indicated that the oral absorption of metformin primarily occurs in the small intestine. This could be achieved through the formulation of sustained release matrix tablets. The administration of these sustained release tablets for metformin hydrochloride may decrease the frequency of dosing and enhance patient compliance [8, 9].
Sustained release oral drug delivery systems are designed to keep therapeutic drug levels in systemic circulation for an extended period [10]. Matrix systems represent the most widely employed oral drug delivery methods due to their ease of use, cost-effectiveness, simplicity in production, reduced dosing frequency, improved patient adherence, and efficacy. Within a matrix system, the drug is dispersed as solid particles in a porous matrix made from either hydrophilic or hydrophobic polymers. The properties of these polymers dictate the release of the drug from the tablet. Hydrophilic polymers, including xanthan gum and PVPK30, are utilized individually or in combination to create hydrophilic matrix tablets aimed at achieving controlled drug delivery formulations. These tablets offer a reduced dosage frequency, enhanced compliance, improved therapeutic effects, fewer side effects, better tolerance, and lower treatment costs. The blending of various hydrophilic polymers enhances the physicochemical and release-altering attributes of the resulting polymer, enabling the creation of an optimized controlled-release product, with careful polymer selection aiding in the regulation of drug release profiles. In aqueous environments, hydrophilic matrix systems swell, subsequently forming gels that erode and dissolve. Additionally, these systems can accommodate high drug loads [11].
Hydroxypropyl methylcellulose (HPMC) is a hydrophilic cellulose ether that serves as a widely utilized pH-independent gelling agent in the preparation of controlled-release formulations due to its drug release characteristics [12]. Its non-toxic nature, ease of handling, and no requirement for specialized production technology make HPMC a common choice for materials that retard drug release [13]. The mechanisms involved in drug release from hydrophilic matrices are intricate, as the micro and macrostructure of HPMC in contact with water changes significantly over time. When exposed to gastrointestinal fluids, HPMC undergoes swelling, gelling, and gradual dissolution [14]. This gel subsequently forms a viscous layer that acts as a protective barrier, limiting both the entry of water and the exit of the drug into solution. The dissolution process may be controlled by diffusion, which depends on the molecular weight and thickness of the diffusion boundary layer.
Natural gums are favored hydrophilic polymers due to their affordability and regulatory acceptance. Xanthan gum, a high molecular weight extracellular poly- saccharide, is produced through the fermentation of the gram-negative bacterium Xanthomonas campestris. Xanthan gum is both biodegradable and biocompatible, forming gels in water, making it increasingly recognized for developing matrices that exhibit controlled drug release properties [15-17]. The gel-forming capabilities of HPMC and xanthan gum can be harnessed to create sustained-release dosage forms. The release of the drug in a hydrophilic matrix system occurs sequentially through swelling to form a gel, diffusion of drug molecules, and ultimately, surface erosion of the matrix [16].
Metformin HCl, the sole available biguanide, is the primary drug therapy for individuals with Type 2 diabetes mellitus (T2DM) and operates by reducing hepatic glucose production and enhancing peripheral insulin sensitivity [18]. The benefits of metformin include a very low risk of hypoglycemia, weight neutrality, and a lowered risk of cardiovascular issues and mortality [19]. As an oral anti-hyperglycemic agent, metformin has incomplete absorption from the gastrointestinal tract and an absolute bioavailability of 50-60%, coupled with a relatively short plasma half-life of 1.5 to 4.5 hours [20-21]. A significant challenge in the effective use of metformin therapy is the frequent occurrence of gastrointestinal side effects, including abdominal discomfort, nausea, and diarrhea, particularly during the first weeks of treatment [22]. These side effects, along with the necessity of taking the medication two to three times a day for larger doses, can reduce patient adherence. A sustained-release (SR) formulation that maintains plasma drug levels for 10 to 16 hours may allow for once-daily dosing of metformin. Such SR products are necessary to extend the duration of metformin’s action and enhance patient compliance.
The primary aim of this study was to formulate matrix sustained-release tablets of metformin using natural gums (specifically xanthan gum) as effective hydrophilic matrix systems, in comparison to the extensively studied hydrophilic matrices (hydroxypropyl methylcellulose and PVPK30) regarding their in vitro drug release rates.
Metformin hydrochoride was obtained from Yarrow Chem Products (Mumbai, India). Hydroxypropyl methyl- cellulose K100M and Polyvinylpyrrolidone K-30 and xanthan gum were obtained as a gift sample from Vignan pharmacy college, (Guntur, India). Lactose, magnesium stearate, Talc were obtained from Yarrow Chem Products (Mumbai, India). All other ingredients used were of laboratory reagents and used as such without further testing.
Drug excipient compatibility study:
Infrared spectra of the pure drug and drug with polymers were recorded on a Fourier transform infrared spectro- photometer. The disc method was employed to study possible interactions between drug and selected polymers. Infrared spectrum was taken by scanning the sample in KBr (IR grade) discs and analyzed over a wave number range of 4000-400 cm-1. Transmittance spectra were recorded.
Preparation of metformin hydrochloride matrix tablets:
Matrix tablets containing metformin hydrochloride were formulated using a wet granulation technique with different ratios of hydrophilic polymers either individually or in combination.
The formulation details for the matrix tablets are presented in [Table. 1]. The preparation of metformin HCl matrix tablet formulations was executed through the wet granulation method. Initially, all components except for magnesium stearate and talc were sieved using a 30-mesh sieve. The drug was blended with polymers (HPMC K100M, xanthan gum, and PVP K30), and an isopropyl alcohol-based binding solution was incrementally added to achieve a cohesive wet mass. This wet mass was then passed through a 20–25 mesh sieve and dried at 60°C for 30 minutes. After drying, the granules were sieved again with a 25 mesh sieve, followed by the addition of lubricants (talc and magnesium stearate). Ultimately, the final mixture was compressed into tablets using a rotary press, aiming for a hardness of 5–7 kg/cm².
Micromeritic properties of blended powder [12]:
1. Angle of Repose
The frictional forces in a loose powder can be measured by the angle of repose. Angle of repose is defined as maximum angle possible between the surface of a pile of the powder and the horizontal plane. The angle of repose was determined by the fixed funnel method. Angle of repose was calculated using the following equation.
θ = tan⁻¹(h / r)
Where, θ = angle of repose, h = height of the pile, r = radius of the pile base
2. Compressibility index
The Carr’s compressibility index of the powder blend defines as
CI (%) = [(Tapped Density – Bulk Density) / Tapped Density] × 100
Bulk density is the ratio of mass of powder to the bulk volume. Tapped density is the ratios of mass of powder to the tapped volume.
Evaluation of Matrix Tablets [13]:
The prepared matrix tablets were evaluated for their physical properties like weight variation, hardness and friability and drug content.
1. Weight Variation
Twenty tablets of each formulation were weighed individually using an electronic weighing balance. The average weight was calculated and individual tablet weight was compared with average weight.
2. Hardness friability
The hardness of six tablets was measured by Monsanto hardness tester. Hardness of tablets was measured in terms of kg/cm2.
3. Friability
Friability of tablets was measured by using Roche friabilator. Ten tablets were accurately weighed and placed in the friabilator and operated for 100 revolutions. The tablets were dedusted and reweighed. The friability was calculated using following equation.
| Components (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| HPMC K100 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 |
| PVP K30 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Xanthum Gum | 40 | 30 | 20 | 10 | 5 | 5 | 5 | 5 | 5 | 5 |
| Lactose | 120 | 110 | 100 | 90 | 85 | 75 | 65 | 55 | 45 | 35 |
| Magnesium Stearate | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Talc | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Total tablet weight (mg) | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 750 |
4. Uniformity of Drug Content
Ten tablets were weighed from each formulation and triturated in mortar to a fine powder. Powder equivalent to 500 mg of metformin hydrochloride was extracted in 100 ml of pH 6.8 phosphate buffer and liquid was filtered. The drug content was determined by measuring the absorbance at 216 nm (using UV-Visible spectrophotometer, Lab India) after appropriate dilution with pH 6.8 phosphate buffer. The drug content was determined using calibration curve.
5. In-vitro dissolution study
The in-vitro dissolution study of matrix tablets of metformin hydrochloride was performed using USP type II dissolution apparatus (DBK) at a rotational speed of 100 rpm. In order to simulate gastrointestinal transit conditions, the tablets were subjected to different dissolution media. The dissolution medium used was 900 ml of pH 1.2 buffer for first two hours and pH 6.8 phosphate buffer for next four hours. The dissolution medium was maintained at a temperature of 37 ± 0.50 C. At predetermined time intervals, 5 ml of the sample solution was withdrawn from the dissolution apparatus, and the samples were replaced with fresh dissolution medium to maintain sink conditions. The collected samples were filtered through a 0.45 µm membrane filter and the drug content in each sample was analyzed by measuring absorbance at 216 nm after suitable dilution using UV-spectrophotometer (LABINDIA). Cumulative percentage of drug release was calculated using equation obtained from a calibration curve.
6. In-vitro drug release kinetic study
To study the release mechanism of metformin hydrochloride from the sustained release matrix tablets, the in vitro drug release data was fitted to the following the mathematical models.
|
Zero order |
Qt = Q0+ k0t |
(1) |
|
First order |
log C= log C0 – k1t/20303 |
(2) |
|
Higuchi |
Qt = kh t ½ |
(3) |
|
Korsmeyer – Peppas |
Qt/Qα =kp t n |
(4) |
Where Q0, Qt and Qα are the amounts of drug dissolved initially, at time t and at time infinite. C0 and C are the concentrations of drug initially and at time t, W0 and Wt are the amounts of drug in the pharmaceutical dosage form initially and at time t and k0, k1, kh and kp refer to the rate constants obtained from the linear curves of the respective models. n is the diffusional exponent that characterizes the mechanism of drug release. The values of the coefficient were calculated using regression analysis between log Qt/Qα and log time. The diffusional exponent n value was obtained from the slope of the regression equation and kp was calculated from antilog of the intercept value.
If the value of n for a cylinder is < 0.45 it suggests the Fickian release (diffusion controlled), if n > 0.45 and < 0.89 it is non – Fickian release (diffusion and polymer relaxation), 0.89 is case II release (only relaxation and swelling) and > 0.89 it suggests super case II release (relaxation and erosion) for swellable systems
For cylindrical systems like tablets, the n values of 0.45 and 0.89 represent pure diffusion or erosion release respectively [14].
The powder mixture of all the formulations were evaluated for angle of repose, bulk density, tapped density, compressibility index and their values were shown in [Table. 2]. The bulk densities and tapped densities were 0.318 to 0.415 g/cm3 and 0.377 to 0.480 g/cm3 respectively. Powder blend indicated good flow properties with an angle of repose values ranging from 26.12 to 27.820. The Carr’s compressibility index for all the formulations were found to be less than 17 %, which indicates that the powder mixture has good flow properties. Hausner’s ratio was also calculated, the ration was ranged between 1.15 and 1.19.
Sustained release matrix tablets of metformin hydrochloride were prepared by wet granulation technique. Total ten formulations were prepared. The tablet weight variation, hardness, friability and content uniformity for each formulation are shown in [Table. 3]. The weight variation test indicated that the percentage deviation of all tablet formulations was found to be within pharmacopoeia acceptable limit. The hardness of all the tablets was within the range of 5.06±0.82 to 7.71±0.38 kg/cm2. The percentage weight loss in the friability test was found to be below 0.8 % in all the cases indicated that all the tablets had good mechanical strength. The drug content in all the batches was determined by measuring absorbance of sample at 216 nm using double beam UV spectrophotometer (LABINDIA). The content uniformity among different formulations was found to be higher and the drug content was more than 98% which indicates uniform drug distribution in all the formulations.
The release behaviour of Metformin Hydrochloride from their respective formulation was evaluated using a USP Type II (paddle) dissolution apparatus. The test involved 900 mL of 0.1 N HCl for the first two hours, followed by phosphate buffer (pH 6.8) for an additional four hours.
| Formulation Code |
Bulk Density (g/cm³) |
Tapped Density (g/cm³) |
Carr’s Index |
Hausner Ratio |
Angle of Repose (°) |
|---|---|---|---|---|---|
| F1 | 0.3285 | 0.3927 | 0.1639 | 1.1959 | 27.42 |
| F2 | 0.3614 | 0.4298 | 0.1591 | 1.1891 | 26.29 |
| F3 | 0.3311 | 0.3884 | 0.1477 | 1.1732 | 26.59 |
| F4 | 0.415 | 0.4801 | 0.1357 | 1.1571 | 27.82 |
| F5 | 0.3419 | 0.4005 | 0.1461 | 1.171 | 26.71 |
| F6 | 0.3527 | 0.4184 | 0.157 | 1.1863 | 26.89 |
| F7 | 0.3182 | 0.3772 | 0.1564 | 1.1853 | 26.12 |
| F8 | 0.3451 | 0.4125 | 0.1637 | 1.1957 | 26.53 |
| F9 | 0.3664 | 0.4317 | 0.1515 | 1.1784 | 26.37 |
| Formulation | Average weight (mg) |
Friability (%) |
Hardness (kg/cm2) |
Thickness (mm)* |
Content uniformity (%) |
|---|---|---|---|---|---|
| F1 | 720 ± 0.12 | 0.259 | 5.86±0.58 | 5.68 | 96.65 |
| F2 | 726 ± 0.46 | 0.271 | 6.25±0.63 | 6.01 | 98.54 |
| F3 | 733 ± 0.39 | 0.268 | 5.06±0.82 | 5.45 | 99.23 |
| F4 | 735 ± 0.56 | 0.259 | 5.27±0.16 | 5.39 | 101.38 |
| F5 | 740 ± 0.38 | 0.265 | 6.51±0.16 | 6.04 | 99.02 |
| F6 | 744 ± 0.51 | 0.264 | 7.65±0.26 | 5.35 | 100.23 |
| F7 | 750 ± 0.45 | 0.278 | 7.71±0.38 | 5.63 | 98.74 |
| F8 | 756 ± 0.23 | 0.243 | 6.79±0.51 | 5.71 | 97.56 |
| F9 | 779 ± 0.32 | 0.252 | 5.30±0.97 | 5.54 | 99.45 |
| F10 | 790 ± 0.50 | 0.255 | 6.35±0.99 | 5.25 | 98.56 |
The temperature was maintained at 37 ± 0.5°C and the paddle rotation was set at 100 rpm. At predetermined time points, samples were withdrawn and replaced with fresh medium to maintain sink conditions. The drug content in the samples was analysed at 216 nm for Metformin HCl using a UV-Visible spectrophotometer.
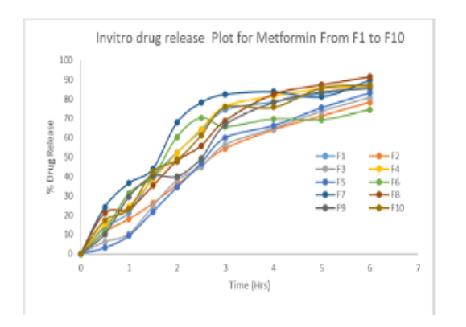
Metformin hydrochloride release from the matrix tablets was studied for first two hours in pH 1.2 buffer and the next seven hours in pH 6.8 phosphate buffer. Total nine formulations were made with Xanthan gum with HPMCK100 and PVPK30 in different ratios. The results of dissolution studies indicated that the Formulations containing HPMCK4M and PVPK30 in combination with xanthan gum released 24.67%, 35.27% and 26.12 % at the end of 2 h and 91.5%, 89.73% and 87.4 % at the end of 9h. Marketed formulation Glycomet from USV showed 28.24% at 2h and 86.48% at 12h.
The comparative dissolution profile of all the formulations is given in [Fig. 1]. The results shown in [Fig. 1] indicate that the release rate of metformin hydrochloride from all the sustained release matrix tablets was dependent on concentration of release retardant contained in the tablet.
The drug release rate was decreased from the tablets as the concentration of release retardant was increase. Xanthan gum alone could not control the release of metformin hydrochloride from the tablets because burst release was observed due to high dose of drug. Two polymers of hydroxyl propyl methyl cellulose, Polyvinylpyrrolidone could control the drug release when combined with high molecular weight xanthan gum up to 6 h. This may be due to the formation of thick gel layer around the tablets by quick hydration of xanthan gum. These results indicated that the Xanthan gum could control the release of metformin hydrochloride up to 6 hrs when it combined with hydroxy propyl methyl cellulose (semi synthetic).
The release data of matrix tablets were fitted into various mathematical models (zero order, first order, higuchi, Peppas) to evaluate the kinetics and mechanism of drug release from the tablets. The model that best fits the release data is selected based on the correlation coefficient value in various models. The model that gives high R2 value considered as the best fit of the release data.
The kinetic parameters for metformin hydrochloride release from the matrix tablets are shown in [Table. 5]. The results indicated that the drug release from the matrix tablets were followed Zero order kinetics. To evaluate drug release mechanism from the matrix tablets plots of percent drug released verses square root of time as per Higuchi’s equation were constructed. The in vitro release profiles of drug from all the formulations could be best expressed by higuchi equation as the plots showed high linearity with correlation coefficient values were in the range of 0.885 to 0.962. Release of the drug from the matrix tablets containing hydrophilic polymers generally follows diffusion. Diffusion is related to drug transport from the matrix tablet into the dissolution fluid. To confirm the diffusion mechanism, the invitro data was fitted into Koresmeyer’s Peppas equation.
For matrix tablets release exponent an ‘n’ value of near 0.68 indicates diffusion control and an ‘n’ value of near 1 indicates erosion or relaxation control. Intermediate values indicates that diffusion and erosion as the release mechanism. The release exponent ‘n’ values were found to be in the range of 0.55 -1.10 indicating that the Non fickian transport is the mechanism of drug release from the matrix tablets.
|
|
Regression (R2) |
K value |
n |
||||
|---|---|---|---|---|---|---|---|
| Zero- order |
First order |
Higuchi |
Korsmeyer |
Zero order mg/hr |
First order hr-1 |
||
| Formulation |
0.934 |
0.985 |
0.962 |
0.956 |
15.40 |
0.186 |
0.682 |
| Formulation code |
Zero -order |
First -order |
Higuchi model |
Korsmeyer Peppas |
||||
|---|---|---|---|---|---|---|---|---|
| R² | k₀ | R² | kₜ | R² | kₕ | R² | n | |
| F1 | 0.870 | 14.80 | 0.907 | -0.229 | 0.943 | 40.91 | 0.941 | 0.796 |
| F2 | 0.963 | 13.28 | 0.932 | -0.190 | 0.958 | 35.19 | 0.987 | 0.827 |
| F3 | 0.954 | 14.41 | 0.884 | -0.197 | 0.929 | 37.79 | 0.955 | 1.107 |
| F4 | 0.872 | 15.0 | 0.899 | -0.215 | 0.946 | 41.49 | 0.954 | 0.761 |
| F5 | 0.949 | 15.26 | 0.930 | -0.212 | 0.911 | 39.72 | 0.949 | 1.359 |
| F6 | 0727 | 11.67 | 0.615 | -0.148 | 0.885 | 34.21 | 0.848 | 0.692 |
| F7 | 0.761 | 13.63 | 0.689 | -0.154 | 0.916 | 39.70 | 0.908 | 0.552 |
| F8 | 0.934 | 15.40 | 0.985 | -0.182 | 0.962 | 41.53 | 0.956 | 0.682 |
| F9 | 0.920 | 14.70 | 0.800 | -0.163 | 0.955 | 39.77 | 0.938 | 0.823 |
| F10 | 0.886 | 14.68 | 0.749 | -0.156 | 0.954 | 40.49 | 0.951 | 0.714 |
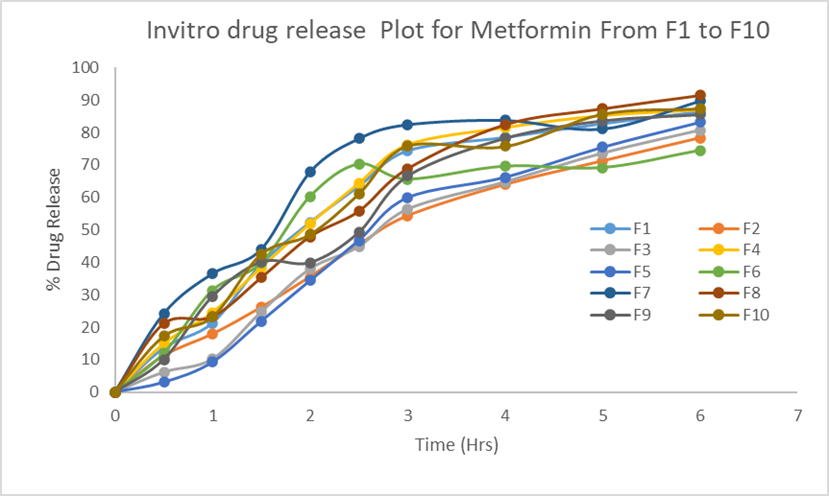
Fourier transform infra-red spectroscopy studies revealed that pure metformin hydrochloride and best formulation showed two typical bands at 3368.99 and 3289.11 cm–1 due to N-H primary stretching vibration and a band at 3151.40 cm–1 due to N-H secondary stretching, a weak intensity band at 1058 cm–1 assigned to C-N stretching vibration and
characteristics strong absorption bands at 1622.33 and 1539.21 cm–1 assigned to C=N stretching vibrations. No significant shifts of reduction in intensity of the FTIR bands of metformin hydrochloride were observed as shown in [Fig. 2]. From the FTIR spectra shown in [Fig. 2], it is very clear that there are no interactions between drug and excipients. All the peaks responsible for the active functional groups were present in the best formulation (F8).
Sustained release matrix tablets containing metformin hydrochloride were formulated using varying proportions of xanthan gum and HPMCK100 alongside PVPK30 through the Wet granulation method. The findings of this study indicate that xanthan gum alone was insufficient in effectively controlling the release of metformin hydrochloride for 6 hours; however, when combined with HPMCK100 and PVPK30, it successfully regulated the release of metformin hydrochloride from the matrices. It can be concluded that a sustained release of metformin hydrochloride was achieved over a duration of 12 hours. The drug release mechanism from the matrix tablets was identified as Release kinetics following Zero order kinetics with Higuchi model diffusion-controlled release mechanism. The sustained release matrix tablets of metformin hydrochloride are anticipated to lessen the frequency of dosing and reduce the dose-related side effects commonly associated with the repeated use of conventional metformin hydrochloride tablets.
The authors are thankful to the management of Sri Venkateshwara university of pharmaceutical sciences, Tirupati, India. for providing necessary facilities to carry out this work.
Declared none.
1. Amitava R, Kalpana R, Sarbani R, Jyotirmoy D, Amitava G, Kazi AA. Response Surface Optimization of Sustained Release Metformin-Hydrochloride Matrix Tablets: Influence of Some Hydrophillic Polymers on the Release. ISRN Pharmaceutics. 2012; 2012 Available from: https://doi.org/10.5402/2012/364261
2. Zubin P, Ronald G, Pamela K. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Canadian Journal of Diabetes. 2018; 42 Available from: https://doi.org/10.1016/j.jcjd.2017.10.003
3. John R, Cesar G, Carolina R, Oswald S. Formulation of a modified release metformin. HCl matrix tablet: influence of some hydrophilic polymers on release rate and in-vitro evaluation. Brazilian Journal of Pharmaceutical Sciences. 2011; 47 (3). Available from: https://doi.org/10.1590/s1984-82502011000300006
4. Rohini D, Alexander S, Chandrasekar MJN. Preparation and in-vitro evaluation of sustained release tablet formulations of metformin HCL. Asian J Pharm and Clinical Res 2012; 5(1): 45-8.
5. Sunil R, Pavan KP, Rajanarayana K, Madhusudan RY. Formulation and release characteristics of a bilayer matrix tablets containing glimepiride immediate release component and metformin hydrochloride as sustained release component. International Journal of Pharmaceutical Sciences and Nanotechnology. 2010; 3 (1). Available from: https://doi.org/10.37285/ijpsn.2010.3.1.8
6. Nanjwade BK, Mhase SR, Manvi FV. Formulation of extended-release metformin hydrochloride matrix tablets. Tropical Journal of Pharmaceutical Research. 2011; 10 (4). Available from: https://doi.org/10.4314/tjpr.v10i4.2
7. Roy H, Chandan KB, Sisir N, Kirti RP. Formulation and design of sustained release matrix tablets of metformin hydrochloride: Influence of hypromellose and polyacrylate polymers. International Journal of Applied and Basic Medical Research. 2013; 3 (1). Available from: https://doi.org/10.4103/2229-516x.112242
8. Karvekar M, Arshad BK. A Brief Review on Sustained Release Matrix Type Drug Delivery System. Journal of Pharmaceutical Research. 2017; 16 (3). Available from: https://doi.org/10.18579/jpcrkc/2017/16/3/118769
9. Jaya S, Srilaxmi G. Formulation and in-vitro characterization of ambroxol hydrochloride sustained release matrix tablets. International Journal of pharma and Bio Sciences. 2019; 10 (3). Available from: https://doi.org/10.13040/IJPSR.0975-8232.10(3).1208-13
10. Chinna Eswaraiah M, Jaya S. Design and in vitro characterization of floating tablets of metronidazole. Asian Journal of Pharmaceutical and Clinical Research. 2019; 12 (3). Available from: https://doi.org/10.22159/ajpcr.2019.v12i3.31018
11. Atif A, Iqbal M, Naveed A, Haji MSK, Aftab U, Minahaj U, <I>et al</I>. Assessment of xanthan gum based sustained release matrix tablets containing highly water soluble propranolol HCL. Acta Poloniac Pharm Drug Res 2013; 70: 283-9.
12. Alderman DA. A review of cellulose ethers in hydrophilic matrices of oral controlled release dosage forms, Int. J. Pharm. 5 (1984)1-9
13. Yan G, Li H, Zhang R, Ding D. Preparation and Evaluation of a Sustained-Release Formulation of Nifedipine HPMC Tablets. Drug Development and Industrial Pharmacy. 2000; 26 (6). Available from: https://doi.org/10.1081/ddc-100101284
14. Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-Matrices for Controlled Drug Delivery: A New Model Combining Diffusion, Swelling, and Dissolution Mechanisms and Predicting the Release Kinetics. Pharmaceutical Research. 1999; 16 (11). Available from: https://doi.org/10.1023/a:1018914301328
15. Lu MF, Woodward L, Borodkin S. Xanthan Gum and Alginate Based Controlled Release Theophylline Formulations. Drug Development and Industrial Pharmacy. 1991; 17 (14). Available from: https://doi.org/10.3109/03639049109048063
16. Talukdar MM, Plaizier-Vercammen J. Evaluation of Xanthan Cum as a Hydrophilic Matrix for Controlled-Release Dosage form Preparations. Drug Development and Industrial Pharmacy. 1993; 19 (9). Available from: https://doi.org/10.3109/03639049309062999
17. Tobyn MJ, Staniforth JN, Baichwal AR, McCall TW. Prediction of physical properties of a novel polysaccharide controlled release system. I. International Journal of Pharmaceutics. 1996; 128 (1-2). Available from: https://doi.org/10.1016/0378-5173(95)04230-x
18. Gohel MC, Parikh RK, Nagori SA, Jena DG. Fabrication of Modified Release Tablet Formulation of Metoprolol Succinate using Hydroxypropyl Methylcellulose and Xanthan Gum. AAPS PharmSciTech. 2009; 10 (1). Available from: https://doi.org/10.1208/s12249-008-9174-1
19. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. Diabetes Care. 2009; 32 (1). Available from: https://doi.org/10.2337/dc08-9025
20. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil H. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. New England Journal of Medicine. 2008; 359 (15). Available from: https://doi.org/10.1056/nejmoa0806470
21. Dunn CJ, Peters DH. Metformin: A review of its pharmacological properties and therapeutic use in non-insulin dependent diabetes mellitus. Drugs. 1995; 49 (5). Available from: https://doi.org/10.2165/00003495-199549050-00007
22. Defang O, Shufang N, Wei L. In Vitro and In Vivo Evaluation of Two Extended Release Preparations of Combination Metformin and Glipizide. Drug Development and Industrial Pharmacy. 2005; 31 (7). Available from: https://doi.org/10.1080/03639040500216410
23. Jaya S, Amala V. Formulation and invitro evaluation of oral disintegrating tablets of amlodipine besylate. International Journal of Applied Pharmaceutics. 2019; 11 (1). Available from: https://doi.org/10.22159/ijap.2019v11i1.28457
24. Sudipta D, Arnab S, Himadri SD. Formulation, in-vitro release kinetics and stability interpretation of sustained release tablets of metformin hydrochloride. Int J Pharma Sci 2015; 7(3):418-22. 14.
25. Jaya S, Divya S. Formulation and in-vitro evaluation of matrix tablets of metoclopramide hydrochloride. International Journal of Applied Pharmaceutics. 2019; 11 (2). Available from: https://doi.org/10.22159/ijap.2019v11i2.30506
© 2025 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.