

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v21i1.ms21.81
Year: 2022, Volume: 21, Issue: 1, Pages: 27-37
Original Article
Nisha Choudhary 1, Dipika Chavda ✉ 1, Vaishali Thakkar 1, Tejal Gandhi 2
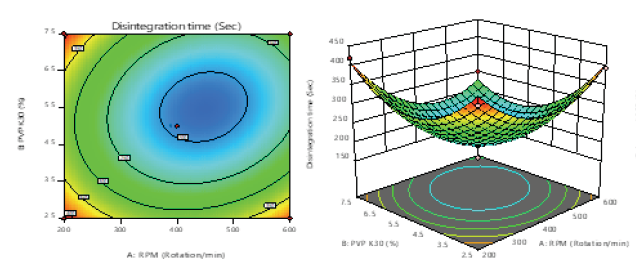
This study was aimed to utilize the Moisture Activated Dry Granulation (MADG) technique to formulate Bicalutamide tablet and identify critical factors influencing its dissolution. The Bicalutamide inclusion complex was formed using the kneading method. Aeroperl 300 was selected as an adsorbent, polyvinylpyrrolidone (PVP) K30 as a binder, Microcrystalline Cellulose (MCC) and Lactose Monohydrate (LMH) in1:1 ratio as fillers. Croscarmellose sodium (CCS) and neusilin were used as disintegrating agents, as they did not affect the disintegration time when hardness and compression force increased. Box Behnken experimental design was used to optimize formulations and was evaluated for pre and post-compression parameters. The optimized formulation was compared with the marketed and wet granulation formulation. In addition, the short term stability testing of the optimized batch was performed. The optimized inclusioncomplex of hydroxypropyl beta-cyclodextrin (HP-ß-CD) was selected based on a phase solubility study in 1:1 ratio with drug toimprove solubility. The optimized batch was prepared by MADG at granulator speed of 540rpm, using 4.30 % PVPK30, and 1.5 % Aeroperl 300. It showed a disintegration time of 208.33 sec.Percentage drug release was 95.02 % in 30 mins, and hardness 5.4 kg/cm2. The stability study results confirmed the stability of the tablets. The Bicalutamide tablet was successfully formulated using the MADG technique. The parameters affecting the in-vitro dissolution were identified and optimized, leading to better bioavailability.
Keywords: Bicalutamide, Moisture Activated Dry Granulation technology (MADG), hydroxypropyl beta-cyclodextrin (HP-β-CD), Box Behnken design (BBD), Croscarmellose sodium
© 2022 Published by Krupanidhi Educational Trust. This is an open-access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.