

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v20i4.MS21076
Year: 2021, Volume: 20, Issue: 4, Pages: 50-57
Original Article
Raja K Rajeswari ✉ 1, A Suneetha 2
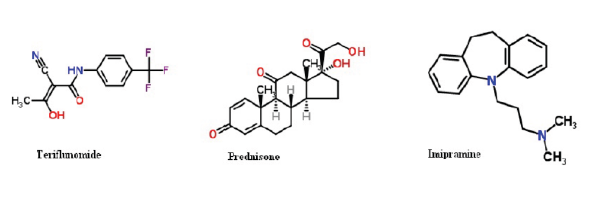
Combination therapy or polytherapy is that uses more than one medication to treat a single disease and associated diseases. Pharmaceutical combination therapy may be achieved by prescribing separate drugs, or, where available, dosage forms that contain more than one active ingredient. A randomized efficacy phase 4 study is going on for prednisone in the treatment of multiple sclerosis, which is a neurodegenerative disease associated with many other disorders. A novel and accurate liquid chromatography tandem mass spectrometry method using electrospray ionization mode has been developed and validated for the simultaneous determination of prednisone (PDN), pioglitazone (PGZ) and teriflunomide (TFM) using imipramine (IMP) and ibuprofen (IBP) as internal standards (IS). The separation was carried on XTerra MS C18 (100 mm x 3.9 mm, 5 µm) reversed phase column using acetonitrile and 0.01M ammonium formate as the mobile phase in gradient mode at 1.0 mL/min. The method was validated in terms of specificity, linearity, accuracy and precision over the concentration range of 1–500 ng/mL. The intra and inter-day precision and accuracy, stability and extraction recoveries of all the analytes were found in the range of 97.2-102.2%. The lower limit of quantitation was 1.0 ng/mL for all the 3 analytes and the extraction recovery values were more than 65%. The method proved highly reproducible and sensitive and was successfully applied to pharmacokinetic study after single dose oral administration of PDN, PGZ and TFM to the rats.
Keywords
Polytherapy, Teriflunomide, Prednisone, Pioglitazone, Phase 4, Multiple Sclerosis, Pharmacokinetics
© 2021 Published by Krupanidhi Educational Trust. This is an open-access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.