

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v19.4.rathod
Year: 2020, Volume: 19, Issue: 4, Pages: 6-10
Original Article
Rathod Viral Jechandbhai1, Amit Kumar Das1,∗
1Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, Karnataka, India
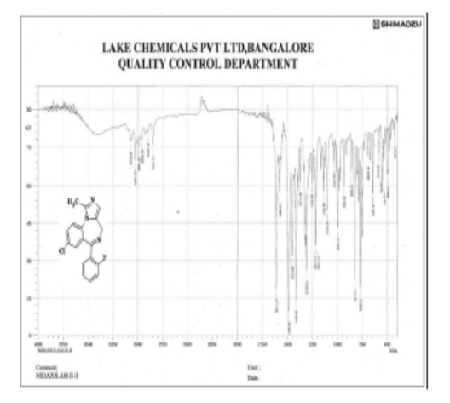
Midazolam is a short-acting benzodiazepine used extensively for sedation and anaesthesia. Conventional synthetic pathways tend to produce varied yields and purities. The current work aimed to optimise the oxidation and purification steps to improve reproducibility, yield, and purity. The objective of this study was to compare the different oxidation and purification techniques used in midazolam synthesis and determine the optimal trial for producing high-purity products by analytical validation. Midazolam was prepared in two stages. Step I involved the condensation of 2-aminomethyl-7-[2-fluorophenyl]-1H-1,4-benzodiazepine dimaleate with triethylorthoacetate in a biphasic medium. Stage II was oxidation using MnO₂ obtained by three procedures (X, Y, Z) and purified using methods (P, Q, R). The products were characterised by TLC (Rf ~0.62), IR spectroscopy, 1H NMR, mass spectrometry (m/z 325), melting point analysis, and high-performance liquid chromatography (HPLC). Trial 8 (Method Z for MnO2 and Method R for purification) yielded a maximum crude yield (92.10%) and pure midazolam base (74.72%) with 99.96% purity. The spectral data established the structure: C18H13ClN3F, MW 325.7 g/mol, and MP 158°C. The combination of MnO2 (Method Z) and purification (Method R) is a reproducible and high-yield method for midazolam synthesis. This method improved the purification of midazolam with high efficiency and product purity.
Keywords: Midazolam, Manganese dioxide oxidation, HPLC, Analytical methods
© 2020 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.