

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v20i4.MS21084
Year: 2021, Volume: 20, Issue: 4, Pages: 43-49
Original Article
Santosh Patil ✉ 1, Rajeev Chadar 1, Ashok Prasad 2, Poonam Koppula 1, Santhosh Koppula ✉ 3
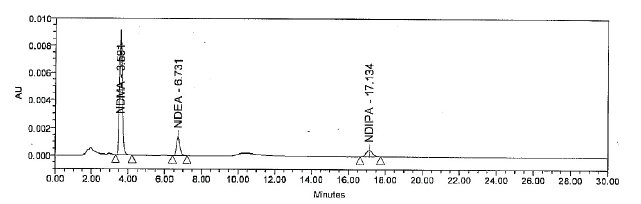
Impurity profiling is an important aspect in drug therapy for its safety and efficacy. The study of impurities of sartans, the first line antihypertensive drugs, has become critical due to presence of cancer causing N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA) and N-nitrosodiisopropylamine (NDIPA) in them, resulting from production and degradation process. These impurities have led to worldwide recall of products. Hence, a simple and accurate method has been developed and validated for simultaneous detection of NDMA, NDEA and NDIPA in Losartan using High Performance Liquid Chromatography - Ultra violet (HPLC - UV) system. The impurities were analyzed on Inertsil ODS 3V (250mm × 4.6mm, 5.0µm) analytical column by using water:methanol (60:40) as the mobile phase at a flow rate of 1.0 mL/min, with a run time of 30 mins. The method was developed for the acceptance limit of 0.64 ppm for NDMA, 0.177 ppm for NDEA and NDIPA respectively. On comparison with existing approaches, the developed method is fast, ideal for routine screening and is suitable for both laboratory and industrial uses.
Keywords: Losartan, HPLC - UV, N-nitrosodimethylamine, N-nitrosodiethylamine, N-nitrosodiisopropylamine
© 2021 Published by Krupanidhi Educational Trust. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.