

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v20i4.suneetha
Year: 2021, Volume: 20, Issue: 4, Pages: 67-70
Original Article
A Suneetha ✉ 1, G I Priyadarshini 1, V Mounika 1, Ayesha Ameen 1, K Jyothi 1, A Babitha 1
1 Department of Pharmaceutical Analysis, Hindu College of Pharmacy, Guntur, Andhra Pradesh, 522002, India
✉ Corresponding author: A Suneetha; [email protected]
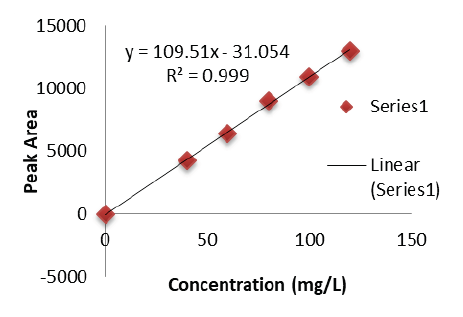
Background: Naloxegol is a peripherally acting µ-opioid antagonist. Purpose: The aim of the present research was to develop and validate a Reverse Phase High-Performance Liquid Chromatography for quantitative determination of Naloxegol in pharmaceutical dosage forms. Methodology: HPLC system used was Shimadzu coupled to a Photodiode Array Detector and was operated in an isocratic mode. Separation was achieved using Inertsil-C18 ODS column having dimensions 250 mm × 4.6 mm, 5 μm and the mobile phase composed of 90 volumes of methanol and 10 volumes of acetonitrile mixture. The flow rate of the mobile phase was 1 mL min−1. Detection wavelength was 250 nm and temperature was 25°C. Findings: The method was validated with regard to linearity, accuracy, precision, selectivity, and robustness in accordance with ICH guidelines. Conclusion: From this study it was concluded that the proposed method is accurate, reproducible and precise. Application: The method was applied successfully for the estimation of Naloxegol in marketed tablet dosage form.
Keywords
High-Performance Liquid Chromatography, Naloxegol
© 2021 Published by Krupanidhi Educational Trust. This is an open-access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.