

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 2, Pages: 111-118
Original Article
Darshan Neelagund1, Rajesh Kumar Rawri2, C K Prasanna1, Paramita Das2,∗
1Research Scholar, Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, 560035, Karnataka, India
2Professor, Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, 560035, Karnataka, India
*Corresponding Author
Email: [email protected]
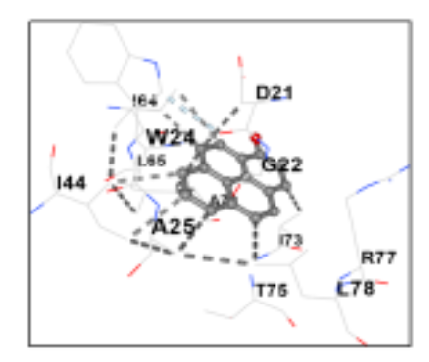
The current work aims to screen the phytoconstitutes of the Lactuca virosa leaves ethanolic extract by using GC-MS analysis and investigate its antibacterial activity. GC-MS analysis was conducted to identify the various phytochemical constituents within the ethanolic extracts of Lactuca virosa. Subsequently, protein-ligand docking was performed using proteins PDBID: 6AHT and 5C5H, revealing a strong affinity between the bioactive compounds and the proteins, indicating potent inhibitory action. Furthermore, each concentration of Lactuca virosa was assessed for antibacterial activity using Minimum Inhibitory Concentration (MIC) against bacterial strains including Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa and Streptococcus mutans. Preliminary phytochemical testing revealed the presence of alkaloids, coumarins, flavonoids, glycosides, phenols, terpenoids, oils, and resins. GC-MS showed the presence of many bioactive compounds in extract. Docking results highlighted that two compounds exhibited the most favourable binding energies of approximately -8.1 kcal/mol and -8.5 kcal/mol with 6AHT, and -8.8 kcal/mol with 5C5H. The ethanolic extract of 0.4mg concentration has shown good antibacterial activity against gram positive bacteria. The study identifies a new source of antibacterial compounds, which could lead to the development of new drugs, particularly effective against gram positive strains like Bacillus cereus and Streptococcus mutans.
Keywords: Lactuca virosa, GC-MS, Docking, ADMET, Antibacterial Activity
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.