

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v22.3.22.6
Year: 2023, Volume: 22, Issue: 3, Pages: 172-177
Original Article
P Seetharamaiah1, Nagaraju Pappula1,*, G Poornima1, G Chandanashree2
1Hindu College of Pharmacy, Amaravathi Road, Guntur, Andra Pradesh, 522002, India
2Department of Pharmaceutics, Krupanidhi College of Pharmacy, Karnataka, Bangalore, India
*Corresponding Author
Email: [email protected]
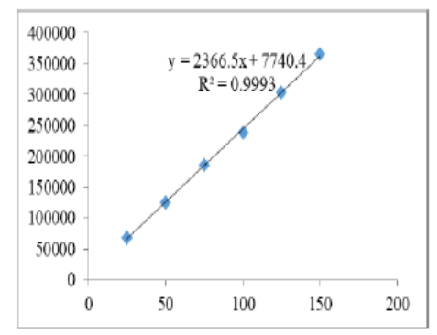
A reliable and exact technique was formulated for concurrently determining Elbasvir and Grazoprevir in tablet dosage forms. Chromatogram was developed by running a sample through Zodiac C18 column (4.6 x 150 mm, 5 µm) with the mobile phase containing Orthophosphoric acid (0.1%) and Acetonitrile in the ratio 50:50 v/v. The solution was pumped through the column at a flow rate of 1 ml/min, while maintaining the column temperature at 30°C. The optimized wavelength selected was 260 nm. The retention times for Elbasvir and Grazoprevir were determined to be 2.32 min and 3.30 min, respectively. The percentage recovery was found to be 100.16% for Elbasvir and 99.49% for Grazoprevir. The LOD and LOQ values obtained from the regression equations for Elbasvir were 0.30 mg/ml and 0.92 mg/ml, and for Grazoprevir were 0.28 mg/ml and 0.86 mg/ml, respectively. The regression equation for Elbasvir was found to be y = 2282.5x + 2407.2, and for Grazoprevir, it was y = 2366.5x + 7740.4. In conclusion, the developed method proved to be simple and economical, demonstrating successful application for the simultaneous estimation of both Elbasvir and Grazoprevir in bulk and combined tablet formulations.
Keywords: Elbasvir, Grazoprevir, RPHPLC, Validation, Simultaneous estimation
© 2023 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.