

Journal of Pharmaceutical Research
Year: 2024, Volume: 23, Issue: 2, Pages: 71-76
Original Article
Shubham N Karle1,∗, Megha T Salve1
1Department of B. Pharmacy, Shivajirao Pawar College of Pharmacy, Ahmednagar, 413725, Maharashtra, India
*Corresponding Author
Email: [email protected]
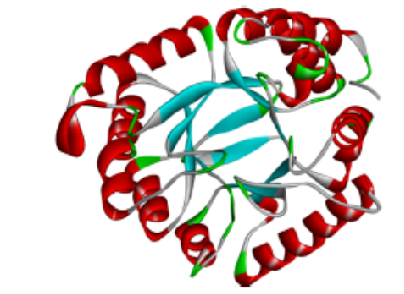
Schiff base-containing (imine or azomethine–C=N–) derivatives have been investigated in relation to a broad range of activity, including antibacterial activity, antiviral activity, anticancer activity, polymer technology and in many other areas due to the presence of moiety in their structures. Antibacterial activity of Schiff bases can achieve by various enzyme inhibitory mechanism. Primary target for the antibacterial drugs is inhibiting dihydropteroate synthase (DHPS) enzyme which result in inhibition of bacterial folate synthesis and act as bactericidal. In this research article Molinspiration software were used to predict pharmacokinetic properties and bioactivity score, in silico docking studies were carried out using 1-click docking software and protox3.0 software were used to toxicity prediction of 10 Schiff base compounds i.e., SB1, SB2, SB3, SB4, SB5, SB6, SB7, SB8, SB9, enzyme (1AJ2) was examined, and possible probability were recorded. and SB10 were carried out against dihydropteroate synthase enzyme (1AJ2) was examined and possible probability were recorded. The purpose of this research is to focus on investigate novel Schiff base derivative by using various computerized software’s to explore their pharmacokinetic properties, bioactivity score, toxicity and docking interaction of ligand and targeted protein.
Keywords
Schiff base, Dihydropteroate Synthase Inhibition, Molecular Docking, ADME Prediction, Antibacterial activity
© 2024 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.