

Journal of Pharmaceutical Research
Year: 2022, Volume: 21, Issue: 3, Pages: 55-69
Review Article
Parin Sidat1,2,*, Malleshappa Noolvi2, Rahul Patil3, Sanket Rathod4
1Ph. D. Scholar, Pharmacy Branch, Gujarat Technological University, Ahmedabad, Gujarat, India
2Department of Pharmaceutical Chemistry, Shree Dhanvantary Pharmacy College, Kim, 394110, Gujarat, India
3Department of Pharmaceutics, Shree Dhanvantary Pharmacy College, Kim, 394110, Gujarat, India
4Department of Pharmaceutical Chemistry, Bharati Vidyapeeth College of Pharmacy, Kolhapur, 416 013, India
*Corresponding Author
Email: [email protected]
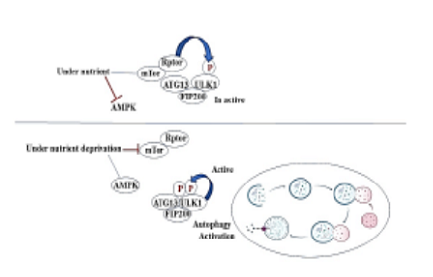
The effectiveness of selective drug therapy for cancer patients has gained much attention from academics and society. However, the rapid development of the drug resistance gained is becoming a significant challenge. As an essential catabolic and homeostatic process, autophagy plays a vigorous role in the degradation or recycling of proteins and cellular components, by which eukaryotic cells recycle or degrade their internal constituents through the machinery of membrane trafficking. Therefore, under traumatic conditions autophagy provides the cells with a safe supply of biomolecules and energy to maintain homeostasis. Deregulation of autophagy pays to tumor genesis, tumor-stromal interactions, and resistance to cancer therapies. Outcome of these interactions between plants and viruses, autophagy performs a vital role in regulating immune-related cell death, antiviral defense, and viral pathogenesis. This study explores the role of autophagy in drug resistance by identifying the mechanism by which autophagy is elaborate in drug resistance, focusing on its mode of action and validation as a potential therapy.
Keywords: Autophagy mechanism; Cancer; ULK1/2 inhibitors
© 2022 Published by Krupanidhi Educational Trust. This is an open-access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
Subscribe now for latest articles and news.