

Journal of Pharmaceutical Research
DOI: 10.18579/jopcr/v18.3.radhika
Year: 2019, Volume: 18, Issue: 3, Pages: 8-12
Original Article
Radhika Majoju1, Mayukh Baidya1
1Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore, Karnataka, India
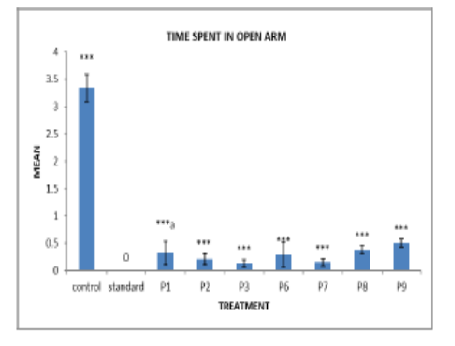
Para-fluorophenyl derivatives are of critical importance in drug development owing to their enhanced pharmacokinetic and metabolic characteristics. This research aims to synthesize and determine the characteristics of new para-fluorophenyl derivatives via acid-amine coupling and Suzuki cross-coupling reactions. The structures of these compounds and their possible. P1 and P2 were synthesized through acid-amine coupling with p-fluoro benzoic acid and lysine and then subjected to BOC protection, deprotection, and hydrolysis. The Suzuki cross-coupling reaction was used to prepare P3 using (4-cyanophenyl) boronic acid. Further derivatives (P4-P9) were prepared via benzoyl, urea, and sulfonyl chloride reactions. The structures were established using melting point analysis, TLC, IR, NMR, and mass spectrometry. Antipsychotic activity in mice was determined using the Elevated Plus Maze method, and statistical analysis was performed using one-way ANOVA and Tukey's test. The synthesized compounds yielded 68-84%, and the spectral data verified their structures. Pharmacological assessment revealed that P1, P3, P8, and P9 were significantly active (P<0.001) as antipsychotics. P1 displayed significant open-arm activity (P<0.05), whereas P3 and P8 exhibited highly significant closed-arm effects (P<0.01). This study introduces new para-fluorophenyl derivatives with improved antipsychotic activities. This new synthetic approach effectively synthesizes structurally varied fluorinated compounds, providing potential candidates for future drug development.
Keywords: Para-Fluoro Phenyl Derivatives, Antipsychotic Activity, Suzuki Cross-Coupling
© 2019 Published by Krupanidhi College of Pharmacy. This is an open-access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Subscribe now for latest articles and news.